Geoscience Reference
In-Depth Information
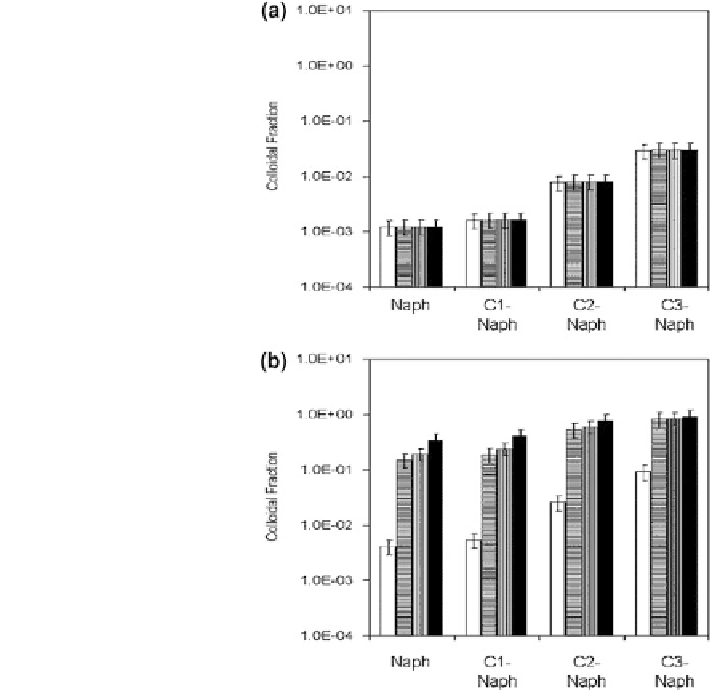
Fig. 8.22 Fraction of
naphthalene concentration
due to colloidal entrainment
at a G
m
= 5s
-1
and b
G
m
= 20 s
-1
, for
naphthalene and naphthalene
compounds containing 1, 2,
or 3 C as side chains, where
G
m
is the mean shear rate.
Reprinted with permission
from Sterling et al. (
2003
).
Copyright 2003 American
Chemical Society
The salting-out effect (see
Sect. 6.5
) may lead to lower solubility of organic or
organo-metallic contaminants in saline waters compared to those obtained in pure
water. The solubility decreases with an increase in salt concentration in water. An
extreme example may be found in Sorensen et al. (
2002
), who examined solubility
of gaseous methane in pure and saline water. Referring to their experimental
results, saline solutions of NaCl (up to 2.5 molality and *11 wt%) were used with
CaCl
2
(up to 2 molality and *20 wt%). Figure
8.23
shows the decrease in sol-
ubility in saline water in the gas-water-salt system. Because methane dissolution
in NaCl and CaCl
2
saline water occurred at different pressure and temperature, the
results cannot be compared directly. However, the decreasing trend is obvious in
both cases.
The electrolyte concentration in an aqueous solvent may affect dissolution of
petroleum products, which are composed of a mixture of hydrocarbons. Dror et al.
(
2000
) considered increasing concentrations of NaCl in water, up to a value
approaching the salinity of seawater, and showed that kerosene dissolution
decreases as the electrolyte concentration in water increases, according to the