Geoscience Reference
In-Depth Information
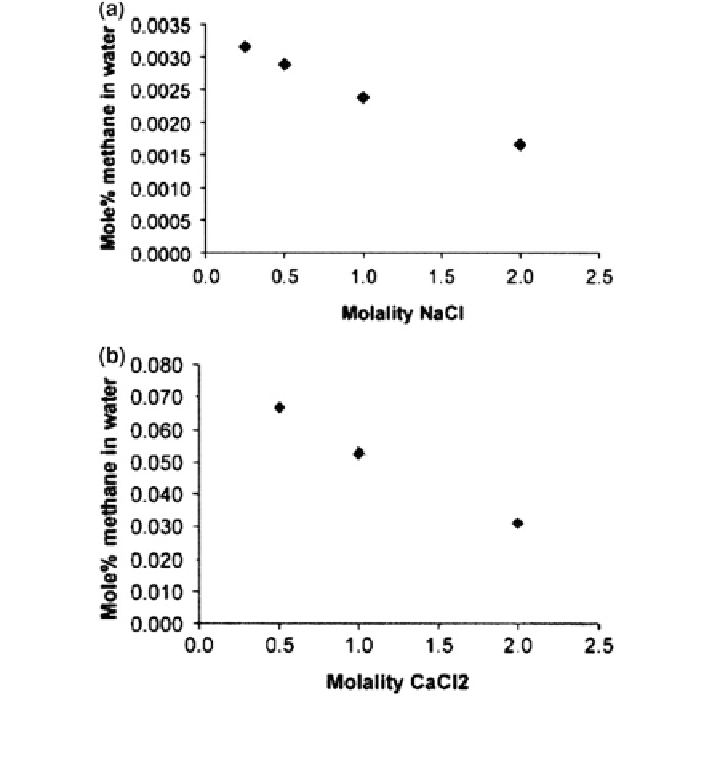
Fig. 8.23 Solubility of methane a in NaCl at 1 atm and 283.15 K and b in CaCl
2
solutions at
37.4 atm and 298.15 K. Reprinted from Sorensen et al. (
2002
). Copyright 2002 with permission
of Elsevier
salting-out effect (Fig.
8.24
). More data on effects of salts on the solubility of a
large number of organic compounds are presented in Table
6.3
.
The partitioning behavior of alkylphenols in crude oil-brine subsurface systems
was reported by Bennett and Larter (
1997
). Partition coefficients were measured in
the laboratory for simulated environmental conditions, from the near to the deep
subsurface, as a function of pressure (25-340 bar), temperature (25-150 C), and
water salinity (0-100,000 mg/L sodium chloride) for a variety of oils. Alkylphenol
partition coefficients between crude oil and brines decreased with increasing
temperature, increased with water salinity and concentration of nonhydrocarbon
compounds in the crude oil, and showed little change with varying pressure. The
results of Bennett and Larter (
1997
) clearly show that, with increasing salt addi-
tion, the partition coefficient of alkylphenols increases, indicating increased phenol
preference for the petroleum phase at higher brine salinity.