Geoscience Reference
In-Depth Information
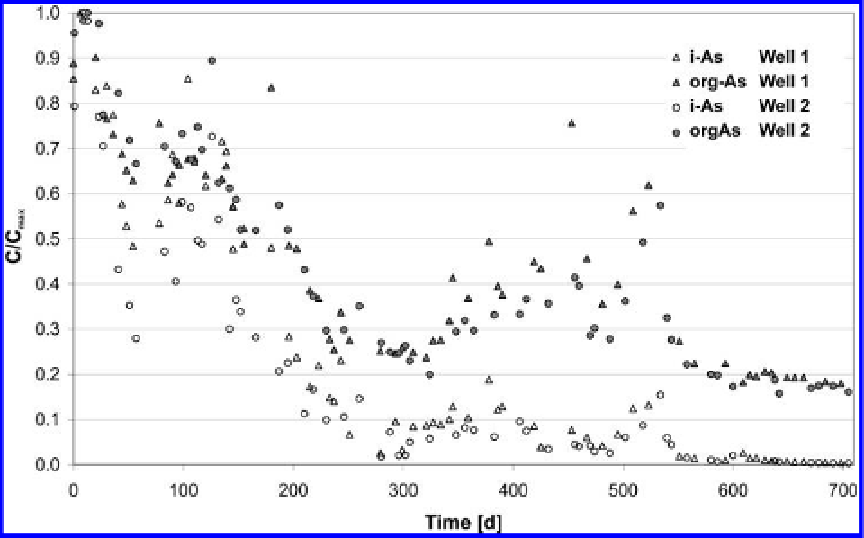
Figure 7.9. Comparing C/C
max
of org-As (grey symbols) and i-As (white symbols) in well 1 (triangles)
and well 2 (circles).
falls below the relative concentration of org-As. At the end of the field experiment the relative
concentration of i-As is 1% and 18% for org-As.
7.4.3
Iron concentration
The Fe concentration in the pumped water is critical for the life expectancy of the remediation
wells. Due to potential clogging problems it is necessary that the Fe concentration is as low as
centrations have been observed during the phase when the plant did not operate correctly. The
concentration reduced directly after the correct schedule of the plant. This can be explained by
the fact that during this period Fe was infiltrated but only a small amount of water with enriched
oxygen was infiltrated. In the absence of oxygen Fe is inhibited from precipitating.
Generally a high fraction of Fe was precipitated: Assuming a uniform distribution of Fe in the
pumped water, a 3 m
3
h
−
1
pumping rate and a 50 mL/cycle dosage the mean concentration in the
water would be 0.26 mg L
−
1
. At 2.8 m
3
h
−
1
pumping rate and 1.000 mL/cycle dosage the mean
concentration would be 5.7 mg L
−
1
. Since the mean Fe concentration that was measured in the
groundwater is significantly below these values it can be assumed that the dosed Fe is reliably
precipitated in the aquifer.
Figure 7.10
shows that Fe was not detectable during the remobilizing experiment between
day 488 and day 533. This behavior was estimated in the first two weeks (day 488 to 501)
since DO-enriched water was infiltrated. However, the Fe-concentration stayed below detectable
limits for the rest of the remobilization experiment after shut-down of this dosing (day 502 to
533). This indicates that the iron hydroxides complexes at which the As is adsorbed are sta-
ble and agrees with literature. E.g., Henning (2004) showed that the initially voluminous iron
hydroxides complexes are dewatered to more compact forms like hematite while they become
older. The compact forms are much more stable compared to the voluminous iron hydroxides
complexes.
Search WWH ::

Custom Search