Geoscience Reference
In-Depth Information
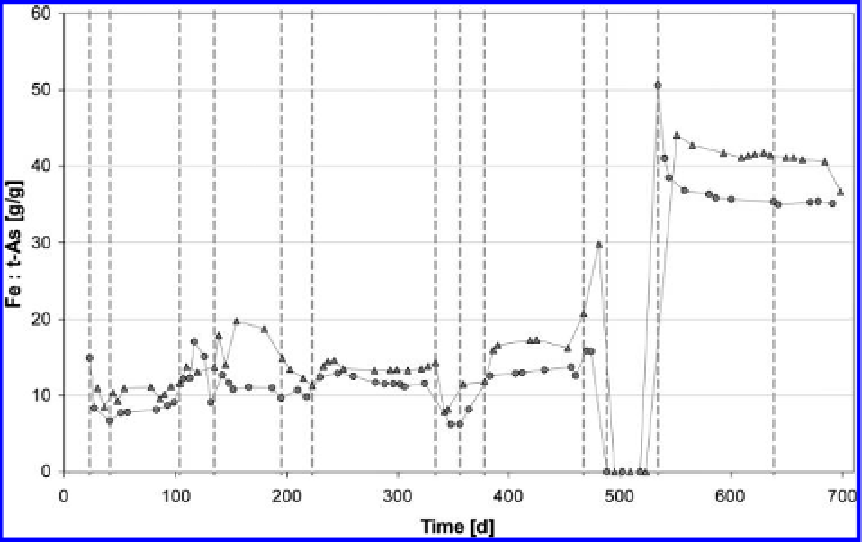
Figure 7.8.
Ratio of dosed Fe to immobilized t-As; triangles denote well 1, circles well 2.
FeCl
2
-dosage system are one explanation of the high ratio of 20:1. After increasing the discharge
from 0.5 to 1.0 m
3
h
−
1
the ratio decreases significantly to about 8:1. This is an analogue to the
calculated rate of the immobilized t-As which was due to the non-stable conditions
(
Fig. 7.7
)
. The
attempt to reach the minimum t-As concentration with high Fe dosage and low discharge showed,
as expected, a high ratio (35:1 to 45:1). This shows that dosing low amounts of FeCl
2
is efficient
in regard of the Fe:As ratio but dosing high amounts of FeCl
2
is efficient in regard of the amount
of removed As.
7.4.2
Change in arsenic species distribution
During the field experiment a speciation of t-As into i-As and org-As has been done following a
method described in detail in Krüger
et al
. (2008) and Holländer
et al
. (2008). At the beginning
of the experiment the fraction of i-As amounted to 11-12% in well 1 and 13-16% in well 2.
With increasing dosage the percentage of i-As decreases. The fraction of i-As in t-As decreased
below 10% during the first three dosing periods. During a break in the plant operation the fraction
increased again. The concentration of i-As decreased temporarily below the detection limit at a
dosage of 500 mL/cycle and 0.5 m
3
h
−
1
discharge. Considering the entire experiment an increase
can be noticed only during the remobilization experiment where the i-As fraction increased from
1.5% to 5%. During the last two dosing periods the concentration decreased below the detection
limit again resulting in an i-As fraction near 0%. The zero value was not reached as the detection
limit was used as the lowest limit in the calculations.
The decrease in the fraction of i-As in t-As implies that i-As is preferably adsorbed to the iron
hydroxide. While the t-As-concentration decreases from 100 to 18% of the initial concentration,
the i-As-concentration decreases from 100% to less than 1%. This was already observed during
the laboratory experiments (Holländer
et al
., 2008). The bulk of the immobilized t-As is org-As
due to its larger amount in the ambient groundwater but i-As is preferentially adsorbed to the Fe.
When comparing the actual concentration of i-As and org-As to their respective initial con-
centrations as done in
Figure 7.9
it can be seen that the relative concentration of i-As quickly
Search WWH ::

Custom Search