Biology Reference
In-Depth Information
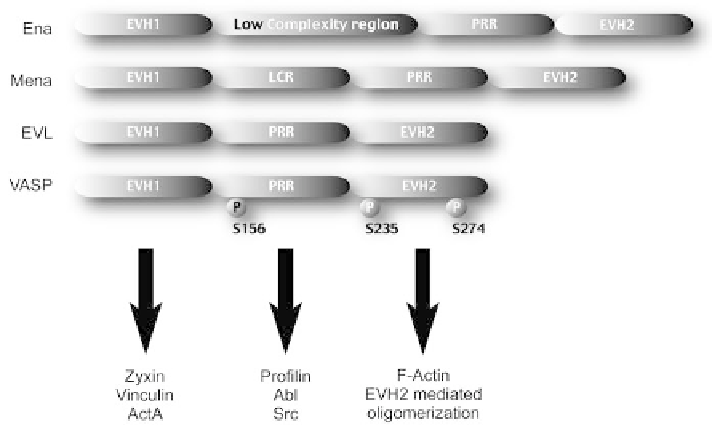
Figure 8.4 The predicted domain structure of Ena (Drosophila enabled), Mena
(Mammalian enabled), EVL (Ena/VASP-like protein) and VASP (vasodilator stimulated
phosphoprotein) showing phosporylation sites on VASP at serines 156, 235 and 274.
EVH1
¼
Ena-VASP homology 1 domain, which binds to ligands such as vinculin, zyxin
and ActA from L. monocytogenes via proline-rich repeats. EVH2
¼
Ena-VASP homology 2
domain, which binds to filamentous actin and also mediates oligomerization of the Ena/
VASP proteins. PRR
¼
proline rich region, which binds to profilin and to Src and Abl
kinases. LCR
¼
Low complexity region
EVL. Ena/VASP family members are largely located in focal adhesions,
cell-cell adherens junctions, tips of stress fibres and the leading edges of
filopodia and lamellipodia, all generally regarded as being sites of active actin
polymerization.
Ena/VASP proteins largely consist of several modular domains (Figure 8.4),
contributing to interactions with many proteins. Principally this is through the
central polyproline-rich region, involved in interactions with SH3 domain-
containing proteins. An important ligand for the proline-rich region is profilin
(Reinhard et al., 1995), an actin monomer-sequestering protein that facilitates
increased actin filament dynamics by increasing the rate of ADP/ATP
exchange on actin monomers and sequestering them in a soluble pool (Wolven
et al., 2000). Mena has been identified as a binding partner for IRS-p53
(Insulin receptor substrate of 53 kDa). Relief of an auto-inhibitory interaction
between the N- and C-terminals of IRS-p53 by binding to activated Cdc42
leads to formation of excessive filopodia which is thought to involve Mena
activity (Krugmann et al., 2001) (see also Chapter 9).
The C-terminal EVH2 domain of Ena/VASP proteins is involved in both
homo- and hetero-oligomerization between members of the family (Bachmann