Environmental Engineering Reference
In-Depth Information
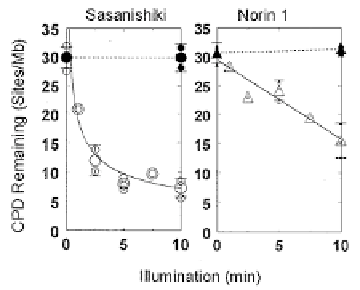
Figure 8. Photorepair (open symbols) and exision repair (closed symbols) in Sasanishiki and Norin 1.
These data implicated a photorepair deficiency in Norin 1, but did not indicate if it
resulted from a regulatory mutation (producing fewer normal photolyase molecules) or
from a structural mutation (resulting in normal numbers of less-effective photolyases).
A powerful method for quantitating enzyme-substrate complex formation in
photolyase reactions is the photoflash technique, pioneered by W. Harm, C.S. Rupert
and H. Harm [5].
Figure 9. Principles of photoflash analysis.
In this method, DNA is irradiated to produce an excess of pyrimidine dimers. Time is
allowed for all available photolyase molecules to bind to a dimer, forming an enzyme-
substrate complex that is stable in the absence of light. Then one intense flash of light is
applied, of sufficient intensity to photolyze all the existing photolyase-dimer complexes,
and of short enough time duration that no photolyase molecules can locate and bind to a
second dimer. Measurement of the number of dimers repaired then gives a direct count
of the number of active enzyme-substrate complexes, and thus of the number of active
photolyases.
Hidema and colleagues probed the nature of this photorepair deficiency in
Norin 1 in two series of experiments using photoflash approaches to "count'' the number
of active photolyase molecules in a cell, to determine the rate of association of the
enzyme-substrate complexes, and its stability. In the first series, the rate of association

Search WWH ::

Custom Search