Environmental Engineering Reference
In-Depth Information
of the enzyme-substrate complexes and the final levels of these complexes were
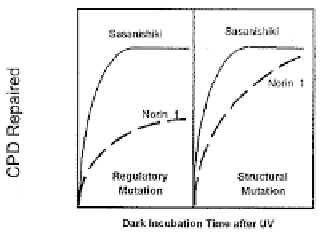
determined: If Norin 1 had a regulatory mutation in photolyase production, the final
level of E-S complexes in Norin 1 would always be less than in Sasanishiki, no matter
how long was allowed for complex formation. However, if it had a structural mutation
Figure 10. Theoretical expectations for photolyase-dimer complex formation in regulatory mutants and in
structural mutants of photolyase.
in the photolyase gene, the normal levels of altered photolyase might have lowered
substrate affinity (manifested by slower E-S complex formation), but given long enough
time, could form the same number of E-S complexes as its UV-resistant relative.
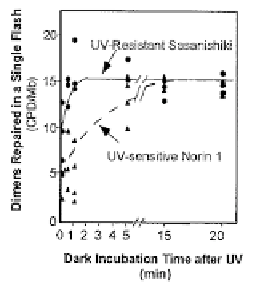
Figure 11. Photoflash analysis of photolyase-dimer complex formation in Norin 1 and Sasanishiki suggests a
structural mutation in photolyase.
The data clearly showed that the latter situation was the case, prevailed, strongly
suggesting that Norin 1 contained a structural mutation in photolyase that affected its
function, not a regulatory mutation that decreased the number of normal photolyase
molecules in each cell.
Hidema et al. wanted to confirm that this was the case, and to exclude the
possibility that some non-repair factor that might slow E-S complex formation in Norin
1. One way to probe this possibility was to determine the properties of the E-S
complex formed in both strains. If the slower photolyase binding (see Figure 11) was
ue to an extrinsic factor, the complexes, once formed, should be similar to those formed
Search WWH ::

Custom Search