Biology Reference
In-Depth Information
intracellular signaling pathways, and compare corresponding
anatomical alterations in axon and dendrites of individual recorded
neurons.
Sections
3
and
4
of this chapter present a detailed discussion
on the techniques of FPR and patch clamp recording; this section
introduces the ways to use these electrophysiological methods to
evaluate synaptic plasticity in CNS injury, with a focus on using
FPR to evaluate short-term and long-term synaptic plasticity.
2. Materials
and Instruments
2.1. Instruments
for Brain Slice
Preparation
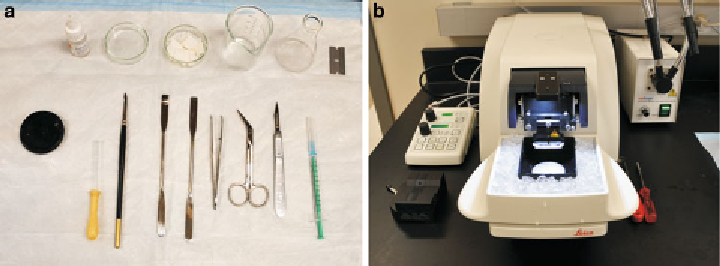
1. Dissecting instruments, including scissors, forceps, scalpel and
blade, spatula, Pasteur pipette, small paintbrush, 50-mm glass
Petri dish, and 50-ml beaker (Fig.
1a
).
2. Vibratome (Fig.
1b
; Leica VT1200, Leica Microsystem).
3. A water bath (preheated to 32°C) holding a submerge cham-
ber for slice incubation (Fig.
2d
).
4. Blades (Gillette) and super glue (Fig.
1a
, b).
1. Sutter P-97 micropipette puller (Fig.
3a
, Sutter Instruments).
2.2. Instruments
for Field Potential
Recording
2. Stereo microscopy (American Scope).
3. DP 304 Differential amplifi er (Warner Instruments).
4. Axon Digidata 1440 A/D-D/A board (Molecular Devices).
5. Analog stimulation isolator (Model 2200, A&M systems).
6. Interface chamber (Fig.
3b
, Warner Instruments, W3 65-0073
and W3 65-0075).
7. Two micromanipulators (Fig.
3b
, Scientifi ca or Warner
instruments).
Fig. 1. Instruments for brain slice preparation. (
a
) Dissecting tools for brain slice preparation. (
b
) A Vibratome (Leica VT1200)
has been set up for slicing.
Search WWH ::

Custom Search