Environmental Engineering Reference
In-Depth Information
PHOTODEMETHYLATION
4
In similar experiments, solutions of MMHg (66-133 µg/L)
were amended with SRFA (1-10 mg/L) and exposed to natu-
ral sunlight (Bergquist and Blum, 2007). Although the rate of
photoreduction was slower (only 20% reduction after 6 hours
of incubation), a larger Rayleigh fractionation as observed:
202/198
2
0
0.9983 to 0.9987 for MMHg versus
0.9994 for Hg(II). Again a signifi cant MIF was detected.
α
product/reactant
-2
-4
MICROBIAL REDUCTION
-6
Microbial reduction of Hg(II) is an alternative pathway
of adding to the pool of Hg(0) available for atmospheric
transport. By exposing bacteria capable of expressing the
enzyme mercury reductase (MerA) to high concentrations
of Hg (NIST SRM 3133), elemental Hg was volatilized and
subsequently trapped in an acidic permanganate solution
(Kritee et al., 2007). The isotopic composition of the growth
medium as well as the volatilized Hg(0) product were mea-
sured. Regardless of the experimental conditions, the bac-
teria preferentially reduced the lighter Hg isotopes. In all
cases, Hg underwent Rayleigh fractionation. The authors
estimated
202
-8
-8
-6
-4
-2
0
2
4
201
Hg (‰)
Δ
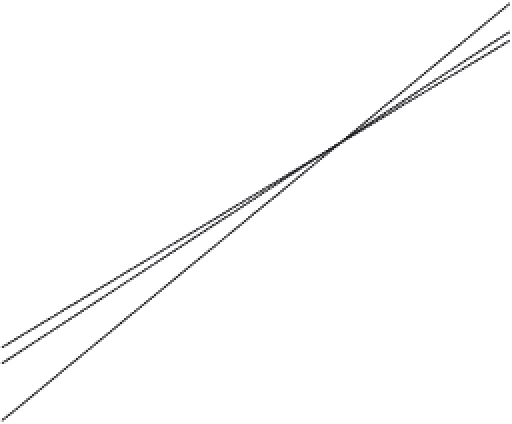
FIGURE 4.3
199
Hg for photochemical reduction of
Hg(II) and MeHg. Positive data are for Hg remaining in solution and
negative values represent reduced Hg(0). Open squares (MeHg) and
open circles (Hg(II)) depict results obtained by. Thin and bold
crosses summarize results obtained in the author's laboratory. The
slopes are 1.36 for MeHg, 1.00 for Hg(II) and 1.26 for the Hg(II)/
Hg(0) data.
199
Hg and
values ranging from 1.0013 to 1.0020. The
overall observed fractionation will be the net effect of sev-
eral steps, including diffusive transport of Hg(II) across the
outer membrane, MerT- and MerP-mediated active trans-
port of Hg(II) through periplasm and the inner membrane,
and fi nally reduction by MerA. Nevertheless, the magni-
tude of fractionation was consistent and independent of
temperature (22 to 37°C), reactor size and type of culture
(pure and natural consortium). Although enzyme-catalyzed
reactions normally lead to increased fractionation at lower
temperatures, the authors speculate that slow transport
through membranes compensates for the expected, but not
observed, increased fractionation. The study did not report
or observe any MIF.
α
Figure 4.3 revealing not only strong fractionation caused
by the photoreduction, but also a signifi cant MIF. After 10
hours of incubation, the remaining Hg was depleted with
light isotopes, showing a
0.46‰, while the
reduced Hg was enriched with light isotopes at
δ
202
Hg of
0.80‰.
The even isotopes of Hg were preferentially reduced result-
ing in an even greater enrichment of
199
Hg and
201
Hg in
solution. The corresponding
199
Hg and
201
Hg values were
1.77 and
2.22‰, respectively, for Hg remaining in solu-
7.94‰ for the photoreduced Hg.
Other investigators (Bergquist and Blum, 2007) amended
Hg in solution (100 µg/L) with Suwannee River Fulvic Acid
(SRFA) (1 mg/L) and obtained reduction rates of
tion, and
6.43 and
90%
after exposing the sample to 6 hours of natural sunlight.
Unfortunately, this study did not provide a mass balance,
so it is unknown whether the loss was entirely due to reduc-
tion. Nevertheless,
CHEMICAL (ABIOTIC) REDUCTION
202
Hg values of 1.67‰ were obtained
at the end of their incubation. This high value is probably
explained by a small residual fraction of Hg, which leads
to a large enrichment of heavy isotopes in Rayleigh-type
systems.
δ
A fractionation factor for the abiotic reduction of Hg(II) by
chemical reagents was determined by adding substoichio-
metric amounts of Sn(II) to Hg(II) in solution (Zheng and
Hintelmann, 2010a). The instantaneously produced Hg
vapor was purged from solution and trapped in acidic per-
manganate. Both, the Hg in the trapping and the remain-
ing solution were measured for Hg isotope ratios. As was the
case with the other reduction processes, lighter isotopes were
preferentially reduced, resulting in
199
Hg and
201
Hg were of similar magnitude,
2.13 and 2.14‰, respectively, resulting in a ratio of
approximately 1.0 between the two MIF processes in the
latter study, while the former experiment using natural
DOC sources gave a ratio of 1.26. Subsequent investigations
observed that the nuclear volume effect contributes to odd
isotope anomalies during abiotic reduction in the absence
of light (Zheng and Hintelmann, 2010a) and identifi ed a
characteristic fractionation pattern for different pathways
of Hg(II) reduction by low-molecular-weight organic sub-
stances (Zheng and Hintelmann, 2010b). Clearly, more
work is required to fully understand the controlling forces
behind MDF and MIF during photoreduction.
at
202
Hg of 5.58‰ in the
remaining solution when 99% of the initial Hg was reduced.
The process followed a Rayleigh-type fractionation with
202
δ
α
1.0011. Only MDF was observed during abiotic reduction.
Evaporation and Volatilization
A few studies have been conducted to compare the frac-
tionation of Hg(0) between the gas phase in contact with