Environmental Engineering Reference
In-Depth Information
the liquid metal and determined a
202/198
Hg
vap/liq
fraction-
2.9700
MeHg
Hg(II)
ation of
0.86
0.05‰ (Estrade et al., 2008) and
0.93
0.17‰ (Sonke et al., 2008)—that is, the vapor phase in
both studies was enriched with lighter isotopes relative to
the liquid metal.
The evasion of reduced Hg in the form of gaseous
mercury from surface waters, such as lakes and oceans
is deemed to be a major pathway of delivering geogenic
Hg to the pool of atmospheric Hg. The process itself is
expected to be purely physical. After reducing Hg(II)
in solution using stannous chloride, evaporation of the
formed Hg(0) was followed over time (Zheng et al., 2007).
The gaseous mercury was collected in acidic permanga-
nate traps and Hg isotope ratios in the volatilized Hg(0)
were compared to the ratio of Hg(0) isotopes remaining
in solution. The pattern during volatilization followed a
Rayleigh fractionation with an observed maximum
2.9675
2.9650
2.9625
2.9600
2.9575
2.9550
B1
B2
B3
B4
C1
C2
C3
F1
F2
F/w1
SRB culture
202
Hg for Hg methylation experiments with
D.
desulfuricans
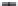
in various media. Circles indicate the isotope ratios
of MeHg produced by the bacteria and squares represent the Hg(II)
starting material. Error bars represent 2 SD of 3-6 replicate measures
from a single culture assay.
FIGURE 4.4
δ
202
Hg
of
1.48‰. As expected for a purely physical process,
volatilization enriched the light isotopes in the gas phase.
The experiments fi t perfectly to a Rayleigh system with
202
α
1.00044 to 1.00047.
SRB grown in media B, C, F, and F w/SO
4
, respectively. For
the
D. desulfuricans
strain, the corresponding
202
Hg values
Methylation
are
1.10‰
0.53,
0.96‰
0.09,
1.05‰
0.30, and
1.18‰ (Dzurko, 2006).
Judging by these preliminary data, it appears that
microbial methylation imparts a fractionation between
Currently, it is unknown whether or to what degree the Hg
isotope ratio signature is altered when Hg(II) is converted
to MMHg. This is crucial information to link Hg sources,
which are commonly dominated by inorganic Hg to Hg in
biota, which is predominantly MMHg, at least in fi sh. A
method was developed to determine compound-specifi c
Hg isotope ratios (Dzurko et al., 2009). The authors applied
a novel “reference standard addition” technique to deter-
mine the isotope fractionation during microbial methyla-
tion. An inorganic mercury standard was added to pure
cultures of the sulfate-reducing bacteria (SRB)
Desulfovibrio
sulfuricans
and
D. propionicus
, which were grown in four
different media (Postgate's Media B and C for growth of
SRB and a medium for fermentative growth of SRB, with
and without addition of sulfate) and the produced MMHg
was isolated from the culture by atmospheric pressure dis-
tillation. Prior to the subsequent analytical procedures, the
same inorganic Hg standard that was used in the methyla-
tion assay was again added to the isolated MMHg. During
the following online separation/determination of MMHg
and inorganic Hg, it was possible to determine the Hg iso-
tope ratio of the original Hg(II) starting material and the
methylated product in a single run. The Hg isotope ratio
deviations between the reactant and the product imme-
diately revealed the fractionation during microbial meth-
ylation. Figure 4.4 illustrates the results obtained for
D
.
desulfuricans
. While the individual absolute isotope ratios
fl uctuate from day to day, the deviation between Hg(II)
reactants and MMHg products is remarkably consistent, as
expressed by the
1.4‰ relative to the Hg(II) starting material.
The variation in isotope fractionation among different
cultures with the same growth medium was too large to
observe any effect of the growth medium on the degree
of fractionation. On average, methylation by the
D. propri-
onicus
and
D. desulfuricans
strains caused a fractionation of
1.12
0.8 and
0.1‰, respectively. Data regard-
ing MIF were inclusive. Because of the relatively large
uncertainty of the chromatographic method, no signifi -
cant MIF was detected.
0.27‰ and 1.07
Binding of Monomethylmercury to Proteins
ATP-synthesizing creatine kinase (CK) is a phosphorylat-
ing enzyme that is strongly inhibited in the presence of
MMHg. By studying the effect as a function of the Hg iso-
tope binding to the enzyme, it was found that magnetic
Hg isotopes were much more effi cient inhibitors than non-
magnetic nuclei (Buchachenko et al., 2004). The authors
postulated that the reaction between CK and MMHg is
spin selective—that is the active site of the enzyme gener-
ates ion-radical pairs with MeHgCl and cysteine residues as
reaction partners, causing mass independent isotope frac-
tionation. The data are reproduced in Table 4.5 and were
used to calculate
201
Hg. According to
these results, the CK reaction would generate an unheard
of mass independent isotope fractionation of several per-
cent. Regrettably, the authors do not present quality-control
data to assess the quality of their isotope abundance
202
Hg,
199
Hg, and
202
Hg.
For the
D. proprionicus
strain, the
δ
δ
202
Hg values are
1.41‰
0.41,
1.25‰
0.16,
0.78‰
0.20, and
1.05‰ for