Agriculture Reference
In-Depth Information
F
3
C
OH
OH
CO
2
H
CO
2
H
O
O
45
46
OH
OH
CO
2
H
CO
2
H
O
O
47
48
CO
2
H
OH
CO
2
H
O
H
O
49
O
Fig. 1.11
The C-8′ and C-9′ modified ABA analogues and (1′
S
, 2′
S
)-2′,3′-dihydro-ABA
with the structural requirements for ABA activity in bioassays, discussed above.
Nevertheless, some exceptions exist. The most significant inconsistency is
observed in C-4′. As described above, 4′-octyl-ABA and 4′-benzyl-ABA (
39
and
40
) retain ABA activity. In the crystal structure of the PYL-ABA-PP2C com-
plex, the C-4′ ketone of ABA forms an indirect hydrogen bond via a water mol-
ecule with the indole moiety of Trp in PP2C. In addition, the gate Pro in PYL
is close to the C-4′ ketone. Thus, the introduction of a large substituent at C-4′
must impair the function of PYL as an inhibitor of PP2C.
n
-Octoxy and benzy-
loxy groups, with lengths of
∼
10 and 5 Å, respectively, seem to be too large to
fit into the space around C-4′ of ABA in the PYL-ABA-PP2C complex. In this
complex, the distance between the 4′-carbonyl oxygen of ABA and the indole
nitrogen of Trp in PP2C is calculated to be approximately 4.5-5 Å, based on the
crystal structures (Fig.
1.12
).
Recently, Takeuchi et al. (
2014
) reported an ABA analogue, AS6, that acts as
an inhibitor of PYL proteins. This molecule has an
S
-hexyl group at C-3′ and is an
effective gate-closing promoter of PYL. Crystallographic data of PYR1 bound to
AS6 demonstrate that the long
S
-hexyl chain of AS6 protrudes through a solvent-
exposed tunnel to prevent PYL-PP2C interactions.





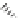
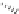


















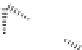





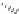







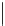

Search WWH ::

Custom Search