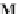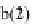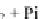Biology Reference
In-Depth Information
a
b
Fig. 11.31 A simplified version of the
conformon mechanism of muscle contraction
first proposed
in Ji (1974b) on the basis of the generalized Franck-Condon principle (Sect.
2.2.3
). (a)A
schematic representation of one turnover of the actomyosin system. A
1
, A
2
, and A
3
are the actin
monomers that are linearly arranged, the center-to-center distance between adjacent actin
monomers being about 5 nm. M stands for the myosin head (also called the S-1 subfragment of
myosin) bound to an actin (A) through non-covalent bond indicated by a vertical bar, |. The figure
indicates that the hydrolysis of one molecule of ATP to ADP and Pi results in the movement of the
myosin head from A
1
to A
3
across a distance of about 10 nm or 100
˚
.(b) The muscle contraction
in the parentheses refer to the states of the actomyosin system that have been actually measured
(see Fig.
11.33
d) using the single-molecule manipulation techniques described in Fig.
11.32
.
Step 1
The actin (A
1
) bound to myosin (see State
a
) is displaced by ATP to produce the myosin-
ATP complex (see State
b
). Step 2
¼
The myosin-actin complex fluctuates between two confor-
mational states - the ground state,
c
, in which the potential energy is stored in ATP, and the
energized state,
d,
in which the potential energy is stored in mechanical strains or conformons
(denoted as two superscripts
*
, each symbol indicating one conformon). In other words, it is
assumed that the hydrolysis of one molecule of the bound ATP to ADP and Pi generates two
conformons in this step. Step 3
¼
One conformon is used to translocate the myosin head from A
1
to A
2
, with a concomitant release of ADP into the medium. Step 4
¼
The second conformon is
used to translocate the myosin head from A
2
to A
3
, releasing Pi into the medium
¼
Each of the steps shown in Fig.
11.31b
can be expanded using the
pre-fit
(or
(B) (1) may implicate the following processes: M•A
1
+ ATP
M
0
+ A
1
+ ATP
⇄
M
0
•ATP + A
1
, where M
0
represents a high-energy (or thermally excited) confor-
mation whose shape is complementary to that of ATP. To account for some of the
unexpected observations reported by Ishijima et al. (1998), e.g., the negative
time values in the histogram of the temporal relation between actin displacement
and the release of nucleotides from myonsin (see Fig. 7 in the above reference), it
may be necessary to invoke a role of the pre-fit mechanisms in the operation of the
actomyosin motor. More specifically, M
0
may have a long enough lifetime (without
violating the Second Law of thermodynamics) to begin to exert force on the actin
filament before receiving free energy input from ATP hydrolysis, thus accounting
for the negative time values in Fig. 7 of Ishijima et al. (1998).
⇄