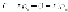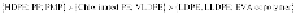Environmental Engineering Reference
In-Depth Information
center. Crystalline fractions act as reinforcing fillers contributing tothehigh
modulus and strength of such materials. Fully amorphous polymers can be
identified experimentally as they do not show an X-ray diffraction pattern
nor a first-order (melting) transition on heating in differential scanning
calorimetry (DSC), both characteristic of crystallinity.
The densities of the crystalline region
ρ
c
and amorphous regions
ρ
a
are very
different. The density of the polymer,
P
, will therefore depend on its percent
crystallinity
x
:
Polymers such as low-density polyethylene (LDPE) where the molecules are
branched are difficult to crystallize compared to high-density polyethylene
(HDPE) and typically have only 35-55% crystallinity. Virtually unbranched
longer molecular form of PE, HDPE, typically has a percent crystallinity as
high as 85% and is used in applications that demand high strength. Percent
crystallinity can be further increased by uniaxial drawing of polymer or by
slow heating below the glass transition (annealing).
The percent crystallinity of commodity plastics generally increases as
follows:
For engineering thermoplastics, the order is as follows:
where UHMWPE is ultrahigh-molecular-weight polyethylene; PB,
polybutene; PBT, poly(butylene terephthalate); PMP,
poly(4-methylpentene); VLDPE, very-low-density polyethylene; LLDPE,
linear low-density polyethylene; and EVA copolymer, (ethylene vinyl
acetate) copolymer.
As a general rule,
atactic
polymers are of too irregular geometry to
crystallize. Plastics such as PS, PVC, and nylon are amorphous; some
amorphous polymers such as polycarbonate (PC), however, can be made
partially crystalline by slow annealing. PE, PP, and PET are partially
crystalline and are generally difficult to get in 100% amorphous form. Their
degree of crystallinity depends on their thermal history as well. A polymer
that is structurally amenable to crystallization, when heated into a melt and