Environmental Engineering Reference
In-Depth Information
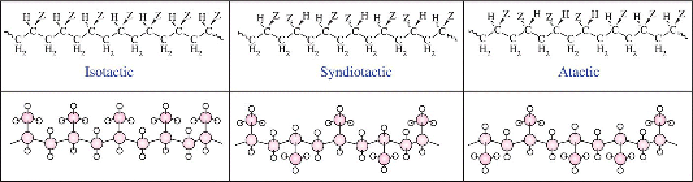
Figure 3.5
Illustration of the stereochemistry in a vinyl polymer. Below
each structural formula is an illustration of the stereochemistry with a “ball
and stick model” for polypropylene.
For instance, the Tg of PP depends strongly on its tacticity.
Atactic
PP has
a Tg ~ −19°C and is used in bitumen and hot-melt adhesive formulations,
while for
isotactic
PP, it is approximately −8°C. The latter is a tough,
semicrystalline plastic used in packaging films. The Tg of s
yndiotactic
form
of PP can be as high as 130°C! Sophisticated catalyst systems even allow
synthesis of polymers that have ordered blocks of
atactic
materials and
isotactic
materials on the polymer chains.
3.2.3 Partially Crystalline Plastics
Polymer chains are too long to be arranged into discrete single crystals.
Single crystals of polymers can only be formed in very dilute solutions in
the laboratory, but these have no particular practical significance in plastics
technology. But when bundles of chains align closely with each other, short
segments of polymer chains can form small highly-ordered regions given
their strong interchain attraction. This yields small crystalline phases
embedded in the surrounding amorphous polymer matrix. These form into
crystalline domains with short-range 3D order and yields a
partially
crystalline
morphology. Polymers are therefore uniquely capable of
“fractional crystallinity” that can vary from a few percent to as high as
90%. The crystallites have a size distribution but are always embedded in
and covalently linked to the amorphous matrix around them (Flory, 1962;
Painter and Coleman, 1997; Strobl et al., 1980).
When these crystallites grow out symmetrically from a nucleating point,
they assume a spherical shape and are called “spherulites.” Still, even within
a spherulite, an amorphous disordered phase can be trapped in, particularly
as intercalated material between the lamellar crystallites radiating from its