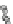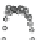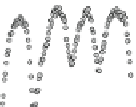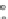Chemistry Reference
In-Depth Information
-10
-5
0
5
10
-10
-5
0
5
10
1,00
1,00
0,99
0,99
0,98
0,98
77 K
300 K
1,00
1,00
V [mm/s]
0,99
0,99
0,98
0,98
-10
-5
0
5
10
-10
-5
0
5
10
V (mm/s)
V (mm/s)
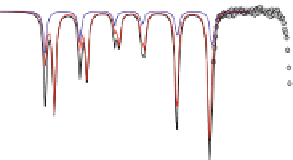
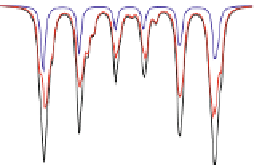
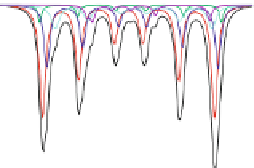
Fig. 4.8 Mösbauer spectra of assemblies of as expected magnetite nanoparticles with two fitting
models: assuming different sextets (low) or ideal maghemite and magnetite phases (high); insert
correspond to a zoom of low energy part of the spectrum (from [
41
])
to the effect of a cationic deficiency in Fe
2+
which are transformed into Fe
3+
,i.e.an
oxidation process which reasonably occurs rather at the surface of the nanoparticles.
Consequently, Mössbauer spectrometry is a priori the most adapted method to evaluate
the exact deviation from stoichiometry d in Fe
3 -d
O
4
from the isomer shift values which
can be used for smaller nanoparticles, i.e. when the superparamagnetic relaxation phe-
nomena occur and prevent from the analysis of broadened lines spectra by means of a 2
components model. As illustrated in Fig.
4.8
, a fitting model which consists thus in
considering the two ideal maghemite and magnetite phases (including the absorption area
of both Fe sites in each component) allows their respective proportions to be estimated
[
41
].Suchamodelling(seeFig.
4.8
) can be also applied to 77 K spectra but remains less
reasonable, because of the rather complex hyperfine structure of magnetite. By means of a
core-shell structural model, one can estimate roughly the thickness of the maghemite
layer, assuming stoichiometric Fe phases. Such assumption remains rather valid for
magnetite nanoparticles prepared by co-precipitation route, after comparing with mag-
netic data which reveal the presence of the Verwey transition, i.e. presence of magnetite
while the Fe
2+
content is decreased as observed by XPS: according to its depth sensitivity,
it confirms that the Fe
3+
species are preferentially located within the surface layer.
It turns out that Mössbauer spectrometry is thus able to establish the mean stoichi-
ometry of as-prepared magnetite-like nanoparticles, to follow the charge ordering and to
appreciate their stability after ageing. According to the literature, one does conclude that
the thickness of the maghemite-like layer is dependent on the morphology and the
synthesis conditions: it is generally ranged at about 2-4 nm [
41
-
43
,
66
,
67
], preventing
thus the existence of pure ultrafine magnetite nanoparticles, except those highly protected
during their synthesis (magnetosome in vesicle and biomineralization in bacterian