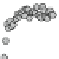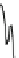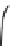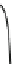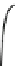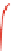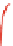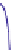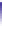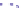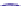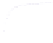Chemistry Reference
In-Depth Information
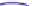
Fig. 4.5 Typical 300 K
Mössbauer spectra of
microcrystalline magnetite
(up) and maghemite (down)
1.00
0.95
0.90
Fe
3
O
4
0.85
1.00
0
0.98
γ
-Fe
2
O
3
0.96
-10
-5
0
5
10
V [mm/s]
attributed to Fe
3+
ions located in tetrahedral site while the inner one corresponds to
both Fe
2+
and Fe
3+
in octahedral sites. The occurrence of a single sextet arises
from the electronic hopping phenomenon between Fe
2+
and Fe
3+
ions with a
characteristic time of *10
-9
s, slightly smaller than the available time for the
Mössbauer measurement. In the case of a stoichiometric magnetite, the hyperfine
parameters are well established: isomer shift relative to a-Fe at 300 K: d = 0.26
and = 0.67 mm/s, quadrupolar shift: 2e = 0.02 and 0.00 mm/s, hyperfine field
B
hf
= 49.0 and 46.0 T for tetrahedral and octahedral sites [
58
], respectively, while
the relative proportions of each Fe species are derived from their respective
absorption area, after correcting the values for the corresponding recoilless factor,
f. Below the Verwey temperature, a partial charge ordering gives rise to a complex
hyperfine structure which can be usually described by means of 4-5 components
attributed to Fe
3+
in octahedral and tetrahedral sites, Fe
2+
in octahedral site and Fe
with intermediate valency states in octahedral sites [
59
].
As illustrated in Fig.
4.5
, the 300 K Mössbauer spectrum corresponding to
maghemite is a sextet resulting from two subcomponents assigned to ferric located
in tetrahedral and octahedral sites but the lack of resolution prevents their
respective proportions to be estimated. Such a situation occurs at low temperature
and an external magnetic field of at least 5 T is necessary to split the hyperfine
structure into two well resolved components, as a consequence of the ferrimag-
netic structure [
60
]. An illustration is given in Fig.
4.6
. But it is important to
mention pioneering studies in 1960s and 1970s carried out on microcrystalline
ferrites [
61
-
65
]. It can be concluded to the following procedure: (1) the modelling
of the in-field Mössbauer spectrum allows the effective field values to be estimated
on both tetrahedral and octahedral sites, together with isomer shift and respective