Biomedical Engineering Reference
In-Depth Information
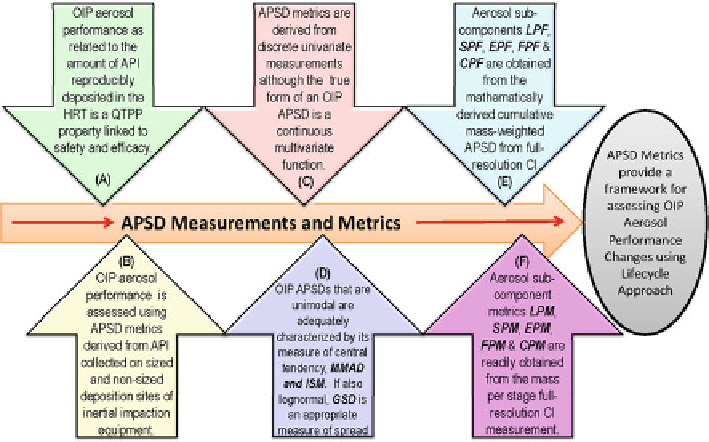
Fig. 5.1
OIP aerosol performance measurements and metrics
5.3
How an AIM-Based Method May Help Simplify the
Process of Determining Metrics Related to OIP APSD
Ideally, a measurement with the relative simplicity of the dose content uniformity
apparatus would be the perfect solution to the problem of reducing the complexity
of the cascade impactor-based measurement process.
In practice, however, at the minimum, it is likely to be necessary to distinguish
between the fi ne mass fraction of particles that have the potential to carry API(s) to
target receptors in the respiratory tract where a therapeutic benefi t may be obtained
[
3
-
7
] and the coarse mass fraction that will likely not penetrate much beyond the
oropharyngeal region [
8
,
9
]. There may even be a requirement to quantify the extra-
fi ne fraction less than ca. 1
m aerodynamic diameter; as such particles are prone to
being exhaled without depositing in the lungs, due to their lack of susceptibility to
the forces governing their movement (predominantly Brownian diffusion) from the
air stream to sites of deposition [
10
].
Early work with a variety of two-stage impaction systems in the 1980s paved the
way towards simplicity in distinguishing solely the coarse from fi ne particle mass
fractions of OIP-generated aerosols [
11
-
13
]. However, in 1992, Miller et al. pointed
out that the Twin Impinger (and by implication, other simple two-portion classifi -
ers) would be incapable of distinguishing unimodal and lognormal APSDs with
particular
MMAD
and
GSD
combinations based on the size selectivity and location
of the collection effi ciency curve defi ning the divide between the two fractions [
14
].
This study was important because it provided a timely warning to those intending to
size-characterize OIP aerosols using such equipment and likely infl uenced the
µ
Search WWH ::

Custom Search