Biomedical Engineering Reference
In-Depth Information
Metal TI
(GSK) throat
DPI Testing
MDI Testing
inlet
cone
pre-separator
lid
Total
aerosol
collection
DPI or MDI
MDI - full apparatus
DPI - full apparatus
Simplified
method
Simplified
method
Stage X
Stage X
MDI MAMass kit
DPI MAMass kit
Fixed sampling
volume option
Impactor or impinger option
CI
pump
Plate
configuration
Dish configuration
(impinger option)
timer
Impactor plates
Flow diverter
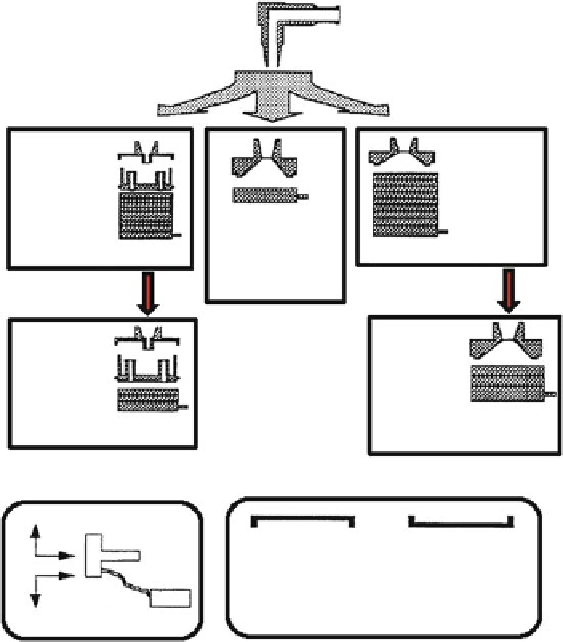
Fig. 5.2
Abbreviated impactor measurement approach to DPI and MDI testing pioneered by Van
Oort and Roberts
(adapted from ref [
15
]—used with permission)
thinking of the regulatory agencies, particularly in the USA. However, they did not
go further by recommending that a two-stage size fractionator might be used in
conjunction with a full-resolution CI system that could be used to verify that the
simplifi ed measurement system was sensitive to the changes in APSD of relevance
to a particular OIP.
Pioneering work by Van Oort and Roberts [
15
] in the mid-1990s pointed the way
forward in terms of setting out a hierarchy of reduced stack Andersen cascade
impactor (ACI) measurements, supported by the full-resolution eight-stage system
(Fig.
5.2
). However, the regulatory approach at that time favored the inclusion of at
least fi ve stages with cut-point sizes between 0.5 and 5.0
m aerodynamic diameter,
and this requirement was a signifi cant contributor to the design of the NGI [
16
].
In the mid-2000s, the abbreviated impactor measurement (AIM) concept and
related effi cient data analysis (EDA) approach were simultaneously developed
out of the need to reduce method complexity. In addition, there was an increasing
recognition by stakeholders involved with the regulatory process for OIPs that
µ
Search WWH ::

Custom Search