Biomedical Engineering Reference
In-Depth Information
Reaction 3.1 The hydriodic
acid-dependent conversion of
methionine to Hcy-
thiolactone (Reprinted from
[68])
N
H
2
NH
2
.
HI
O
HI, 120
o
C
OH
O
-CH
3
I
S
S
CH
3
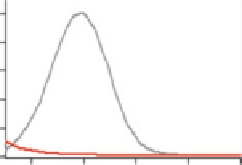
Fig. 3.1 Absorption spectra
of
L
-Hcy-thiolactone
N
H
2
1.0
HCl
(0.2 mM in water, 25
C).
D
,
L
-Hcy (0.2 mM), shown for
comparison, does not
appreciably absorb UV light
above 220 nm (Reproduced
from [68])
O
0.8
S
0.6
NH
3
+
COO
-
HS
220
240
260
280
300
Wavelength (nm)
3.1.2 Acid-Dependent Cyclization of Homocysteine
In the presence of hydrochloric acid,
L
-Hcy undergoes intramolecular condensation
to
L
-Hcy-thiolactone (Reaction
3.2
) [4]. The rate of ring closure depends on the acid
concentration and temperature. For example, in 0.1 N, 0.6 N, or 6 N hydrochloric
acid at 100
C, 50 % of ring closure condensation occurs in 3 h, 15 min, or
5 min,
respectively [68]. Because Hcy-thiolactone, in contrast to Hcy, absorbs ultraviolet
light (Fig.
3.2
), the conversion to Hcy-thiolactone has been exploited in the first
spectrophotometric assay for Hcy [189]. The acid-dependent conversion to Hcy-
thiolactone followed by its quantification by high-performance liquid chromatog-
raphy (HPLC) is now used as a convenient procedure for the determination of Hcy
in biological samples [64, 79, 93-95, 190].
<
3.2 Physicochemical Properties
3.2.1 UV Spectrum
Differences in physicochemical properties of Hcy-thiolactone and Hcy are
highlighted in Table
3.1
. Similar to other thioesters, Hcy-thiolactone absorbs
ultraviolet light with a maximum at 240 nm and
5,000 M
1
cm
1
in water
(Fig.
3.2
) [68]. The ability to absorb at 240 nm is also a characteristic property of a
deprotonated sulfhydryl group [191, 192]. Monitoring absorbance at 240 nm
facilitates studies of nucleophilic [73] and electrophilic [84] reactions involving
Hcy-thiolactone as well as quantification of Hcy-thiolactone in biological samples
[64, 67, 190, 193, 194].
ε ¼



























Search WWH ::

Custom Search