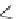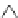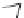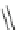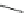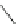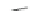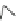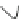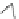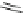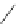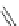Chemistry Reference
In-Depth Information
NN
O
O
N
NN
N
94%
77
31
O
O
O
O
78
79
O
O
R
F
1) NBS,
hv
2) Ni
R
F
N
N
O
R
F
=C
6
F
5
(45%)
C
4
H
2
F
7
(31%)
O
80
81
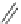
Scheme 22 Synthesis of isocorannulenofuran 77 and imide-fused corannulenes 81 [
107
,
109
,
110
]
lithium [
109
]. Tetrabromocorannulene 18 is a corannuldiyne equivalent. The
remaining two alkenyl moieties in 78 provide the possibility for the further Diels-
Alder reaction with tetracyclone or 77. Electron-poor N-substituted imide-fused
corannulenes 81 were synthesized from fluoranthenes 80 by Method D in
Scheme
6
[
110
].
Fluorine-containing annulated corannulenes 82 and 83 were synthesized by the
reaction of corannulene with a large excess of 1,4-C
4
F
8
I
2
at 300
C, and the former
as the major product was obtained in 15% yield [
111
]. Based on X-ray crystallo-
graphy, the bowl depth of the corannulene core in 82 is around 0.78
, which is
smaller than that of corannulene. Experimental results and theoretical studies
indicated that 82 is a stronger electron acceptor than C
60
.
Å
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
FF
FF
F F
83
82