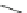Chemistry Reference
In-Depth Information
D
2
, 1 atm
Ni or Rh(I) cat.
67
Δ
H
D
H
D
D
D
H
H
Exo-[D
2
]-
69
Endo-[D
2
]-
69
Scheme 23 Synthesis of
exo
-dideuteriocyclopentacorannulene (
exo
-[D
2
]-69)[
61
]
2.3.2 Structures and Properties of Annulated Corannulenes
Annulation of a five-membered ring to the rim of the corannulene framework
significantly increases the curvature and rigidity of the system. Based on the
crystallographic analysis, both the bowl depth (ca. 1.05
)[
112
] and the POAV
pyramidalization angles (10.7
, 9.7
, and 9.0
) for the hub ring in 67 are larger than
those for the parent compound 1.
Computational studies of the bowl-to-bowl inversion barrier (
Å
G
{
inv
) for 67 and
69 predict values of 27.7-30.9 and 25.6-28.9 kcal/mol, respectively (Table
5
)
[
57
,
113
]. These results are consistent with the fact that 69 has less bowl strain
and is shallower than 67. Heterogeneous or homogeneous deuterogenation of 67
gave
exo
-dideuteriocyclopentacorannulene (
exo
-[D
2
]-69) with the
exo
specificity
(Scheme
23
)[
61
]. The value of
ʔ
ʔ
G
{
inv
for
exo
-[D
2
]-69 was determined to be
ca. 27.6 kcal/mol based on the proton NMR studies.
The inversion barrier of (heterocyclic)
peri
-annelated corannulenes depends on
the bridging groups. The methylene carbon-heteroatom bond length shortens along
the series Se
O and the ring size contracts. This strained
annelation leads to a slight increase in bowl depth, but, due to the high sensitivity
of inversion barrier to bowl depth, there is a significant increase of
>
S
>
C
>
N
>
G
{
inv
.
ʔ
G
{
inv
for the heterocyclic
peri
-annelated corannulenes follows the
Therefore,
ʔ
order: 70
74 (Table
5
)[
57
]. The nitrogen-substituted series
73 was studied in greater detail [
114
].
N
-Phenyl and
N
-butyl substituted
2,3-dihydro-1
H
-corannuleno[2,3-
cd
]pyridines display a value of
>
73
>
72
>
71
>
G
{
inv
of about
16.5 kcal/mol, slightly higher than that of the NH derivative (16.1 kcal/mol).
ʔ
2.4 Benzocorannulene Family
2.4.1 Synthesis
FVP synthesis of benzo[
a
]corannulene 85 was conducted with benzofluoranthene
84 [
115
] or [4]helicene precursor 86 [
116
] (Scheme
24
). The former protocol is
similar to the synthesis of corannulene (1) from fluoranthene 10 (Scheme
2
). In the
reaction with 86,
the reaction mechanism should involve a radical cascade