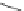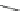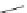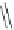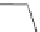Chemistry Reference
In-Depth Information
Table 4 Selected physical properties of multi(phenylthio)corannulenes (Siegel et al., unpublished
results)
Compound
1
60
61
62
63
48 (XR
¼
SPh)
58a
1st Red
CV
[V]
a
2.45
2.43
2.09
2.28
1.95
1.58
1.49
l,a
(nm)
b
ʻ
286
292
295
302
310
344
417
max,f
(nm)
b
ʻ
422
448
456
460
496
492
a
Measured in THF
b
Measured in acetonitrile
SPh
PhS
PhS
SPh
PhS
PhS
SPh
PhS
SPh
60
62
63
61
As described above, stable neutral buckybowl radicals 39 and 40 arise from
hanging relatively stable radical bearing fragments onto corannulene. Charged
o
-semiquinone radical salts are not generally stable; however, the pronounced
stabilization effect arising from highly
tert
-butyl substituted corannulene allowed
the isolation of
o
-semiquinone radical salt 64, which exists for a long time in an
oxygen-free solution and the solid state under a nitrogen atmosphere. Negative
charge densities are delocalized at both of the oxygen atoms and over the whole
curved
network [
104
]. The sum of the absolute spin density on the corannulene
skeleton of 64 (0.880) is larger than that of 39 (0.241) and 40 (0.553). In addition,
the corannulyl backbone provides a suitable bridge for a strong intramolecular
exchange interaction between two radical moieties. Corannulene-based neutral
diradical 65 shows very strong intramolecular antiferromagnetic interaction
through the corannulenyl backbone in the crystalline state, and has a singlet ground
state and a thermally accessible excited triplet state [
105
].
π
Na
+
O
O
MeO
2
C
CO
2
Me
t
-Bu
t
-Bu
t
-Bu
t
-Bu
O
O
t
-Bu
t
-Bu
t
-Bu
65
t
-Bu
64