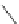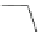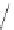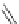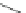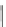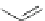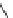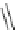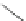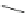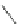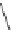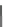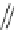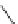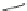Chemistry Reference
In-Depth Information
Br
2
HC
CHBr
2
Zn/Cu
TiCl
4
,DME
Cl
Cl
1000 °C, N
2
Br
2
HC
CHBr
2
10
-
15%
20%
Br
2
HC
CHBr
2
66
68
67
Scheme 18 Synthesis of cyclopentacorannulene (67)[
34
,
106
]
(Me
3
Sn)
2
Pd(PPh
3
)
4
20%
Ni, H
2
100%
or PhLi
20%
BrH
2
C
CH
2
Br
67
69
34
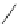
Scheme 19 Synthesis of acecorannulene (69)[
57
,
62
,
106
]
70
(X = O)
71
(X = S)
72
[X = C(CO
2
Et)
2
]
73
(X = NPh)
74
(X = Se)
Br
Br
X
34
70
-
74
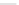
Scheme 20
peri
-Annelated corannulenes (heterocycles) [
57
]
2.3 Cyclopentacorannulene, Acecorannulene and
Annelated Corannulenes
2.3.1 Synthesis
Cyclopentacorannulene (67) was obtained as a mixture with corannulene (in ratio
7:3) in 10-15% yield from dichloride 66 by flash vacuum pyrolysis at 1,000
C
(Scheme
18
)[
106
]. In contrast, 67 could also be prepared, in 20% yield, by
titanium-mediated carbenoid couplings of 68 in solution phase [
34
].
Acecorannulene (69) was generated in excellent yield from cyclopentacor-
annulene (67) by nickel-catalyzed hydrogenation (Scheme
19
)[
106
]. Alternatively,
69 was also synthesized in the solution phase from dibromide 34 by either
Pd-catalyzed Stille-type coupling [
62
] or by treatment with phenyllithium [
57
].
Various (heterocyclic) six-membered
peri
-annelated corannulenes 70-74 were
accessed by the reaction of divalent nucleophiles with 2,3-bis(bromomethyl)-