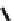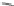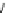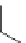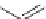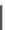Chemistry Reference
In-Depth Information
Me
3
Si
SiMe
3
n
-C
8
F
17
n-1
(
P
)-
78
,n=5
Fig. 22
Structure of acyclic ethynylhelicene pentamer (
P
)-
78
[
72
]
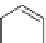
Fig. 23
Structures of
1,12-disubstituted
benzo[
c
]phenanthrenes [
74
]
R R
O
2
S
N
A
D
X=
C
B
XOC
COX
(
P
)-
79a
,R=Me;(
P
)-
79b
, R = Et; (
P
)-
79c
,R=
i
-Pr
H
N
N
H
OEt
O
O
N
N
OEt
2 ClO
4
80
81
,53%
Scheme 16
Synthesis of 1,12-bis(2-pyridyl)benzo[
c
]phenanthrene-2,11-diol
81
[
75
]
The cascade cyclization reactions of the benzannulated enyne-allenes outlined in
Scheme
6
were also adopted for the synthesis of the diindeno-fused 1,12-diphenylbenzo
[
c
]phenanthrene
85
(Scheme
17
)[
76
]. The X-ray structure of
85
(Fig.
24
)showsa
twisted structure with a 59.9
acute dihedral angle between the mean planes of rings
A and D. Each phenyl substituent is oriented at a 60.8
angle from the benzene ring to
which it is attached but is roughly parallel to the opposite side of the twisted benzo[
c
]
phenanthrene framework with a distance of ca. 2.94
˚
at the closest point.
The
1
H NMR signals of the two
ortho
hydrogen atoms on each of the phenyl
substituents of
85
appear at
d
7.88 and 5.71, indicating that the two hydrogen atoms
are in very different magnetic environments and the rate of rotation is relative slow
on the NMR time scale at room temperature. Coalescence of the signals occurs at
ca. 125
C, which corresponds to a rotation barrier of 18.5 kcal/mol. The AB signals
of the methylene hydrogen atoms remain unaffected, indicating a slow rate of
racemization on the NMR time scale.