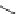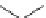Chemistry Reference
In-Depth Information
Fig. 20
Structure of
macrocyclic ethynylhelicene
(
M
,
M
,
M
)-
76
[
65
]
R
R
(
M
,
M
,
M
)-
76
:R=CO
2
n
-C
10
H
21
R
SiMe
3
Me
3
Si
CO
2
n
-C
10
H
21
n-1
77
Fig. 21
Structures of acyclic ethynylhelicene oligomers
77
[
71
]
hetero-double-helix dimer between (
P
)-
78
and (
P
)-
77
(
n ¼
5) is not formed pref-
erentially over the homo dimers. A systematic study of the side chain effect on the
double helix formation has also been reported recently [
73
].
The structure and optical activity of 1,12-dimethyl-, 1,12-diethyl-, and 1,12-
diisopropylbenzo[
c
]phenanthrene derivatives, (
P
)-
79a
,
P
)-
79b
, and (
P
)-
79c
(Fig.
23
), have also been investigated [
74
]. Interestingly, the ethyl and the isopropyl
substituents cause less severe twists when compared to the methyl group. The acute
dihedral angle of the mean planes of the two outer rings (rings A and D) is 47.9
for
(
P
)-
79a
, 45.2
for (
P
)-
79b
, and 41.8
for (
P
)-
79c
.
3.2
1,12-Diarylbenzo[
c
]phenanthrenes
The synthetic strategy outlined in Scheme
5
for 4,5-bis(2-pyridyl)phenanthrenes
28
also found success in the synthesis of 1,12-bis(2-pyridyl)benzo[
c
]phenanthrene
81
(Scheme
16
)[
75
]. Condensation between 4
a
,10
a
-diazoniaanthra[1,2-
a
]anthracene
diperchlorate (
80
) and 1,1-diethoxypropene led to
81
in 53% overall yield.