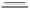
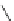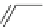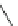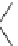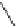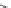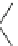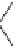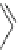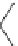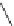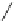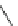Chemistry Reference
In-Depth Information
Scheme 4
Photocyclization
for 4,5-diphenylphenanthrene
[
31
,
32
]
Ph
Ph Ph
Ph
h
n
19
20
, 65%
21
22
23
Br
Br
24
25
Fig. 6
Structures of 4,5-diaryltriphenylenes [
33
]
around the carbon-carbon bonds attaching them to the triphenylene system. At the
coalescence temperature of 16
C, the rotation barrier of
22
was determined to be
15.0 kcal/mol, whereas that of
23
was determined to be 14.8 kcal/mol at 14
C.
Similarly, the rotational barriers of
24
and
25
were determined to be 15.3 and
14.8 kcal/mol, respectively.
The presence of diastereotopic hydrogen atoms on the methylene carbons of
24
and
25
also provided opportunities to determine the activation barriers for racemi-
zation. A barrier of 15.3 kcal/mol was determined for
24
, whereas that of
25
is too
high to be determined by variable-temperature NMR studies. It was estimated that
the activation barrier for racemization is larger than 21.1 kcal/mol for
25
. The
similarity in the rotational barriers of
24
and
25
but very different racemization
barriers suggest a molecular movement in which the phenyl groups turn around
each other like cog wheels for racemization.
Overcrowded 4,5-bis(2-pyridyl)phenanthrenes
28a
and
28b
were prepared from
the Diels-Alder reactions between 4
a
,5
a
-diazoniapentaphene diperchlorate (
26
)
and ketene acetals
27a
and
27b
, respectively (Scheme
5
)[
16
]. The X-ray structure