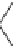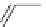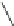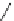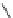Chemistry Reference
In-Depth Information
Fig. 3
Structure of
phenanthrene
4
5
6
3
4a
4b
A
C
2
7
B
1
8
10a
8a
10
9
7
Scheme 1
Synthesis of 4,5-
dimethylphenanthrene [
18
]
NaNH
2
liq. NH
3
Cl
Cl
1
, 90%
8
as ligands to form organometallic complexes for asymmetric catalysis and for chiral
molecular recognition, the configurational stability is of critical importance.
The strain energies in the twisted polyarenes have been determined by compari-
son of heats of combustion with those of the corresponding nonstrained isomers. In
the case of 4,5-dimethylphenanthrene, the comparison was with that of 2,7-
dimethylphenanthrene [
15
]. The bathochromic and hyperchromic shifts of the UV
spectraoftwistedareneshavebeenusedtodetect the presence of twisted distortion [
16
].
2 Twisted Phenanthrenes and Related Compounds
2.1
4,5-Dialkylphenanthrenes
The X-ray structure of phenanthrene (
7
) shows that the aromatic system is nearly
planar with a C4-C4a-C4b-C5 torsional angle (
y
) of only 2.6
(Fig.
3
)[
17
]. The
distortion is more pronounced in 4,5-dimethylphenanthrene (
1
) with a torsional
angle
y
of 31.5
[
5
]. The dihedral angle between the mean planes of the outer A and
C rings (planes 1-2-3-4a-10a and 4b-5-6-7-8-8a) is 27.9
. The strain energy in
1
was determined to be 12.6 kcal/mol from comparison of its heat of combustion with
that of 2,7-dimethylphenanthrene [
15
]. The enantiomers of
1
were separated by
HPLC at cryogenic temperatures (
70
Cto
80
C) on a chiral stationary phase.
{
) for racemization was determined to be 16.1 kcal/mol at
25
C, indicating a rapid rate of inversion of configuration at this temperature [
5
].
The first synthesis of
1
from pyrene was developed by Newman and Whitehouse
in 1949 [
3
]. An alternative synthetic procedure involving cyclization of 2,2
0
-bis
(chloromethyl)-6,6
0
-dimethylbiphenyl (
8
) as a key step was reported in 1979
(Scheme
1
)[
18
]. Other synthetic routes to
1
have also been developed [
19
-
21
].
Several analogs of
1
have been synthesized (Fig.
4
), including 4,5,8-trimethyl-1-
phenanthreneacetic acid (
9a
) bearing a CH
2
CO
2
H group at the C1 position [
22
-
24
].
The presence of the carboxyl group provided a handle to allow partial resolution of
the enantiomers of
9a
, giving the first evidence of a nonplanar structure. Separation
The activation barrier (
DG