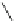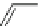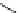Chemistry Reference
In-Depth Information
C12
4
5
3
6
C1
4a 4b
C2
C10A
C10
2
A
C
7
C5
C4A
C6
C4B
C9
C3
B
1
8
C4
10a
C8A
8a
C7
C8
10
9
1
C11
Fig. 1
Structure and ORTEP drawing of 4,5-dimethylphenanthrene [
5
]. Reprinted with permis-
sion from [
5
]. Copyright (1987) American Chemical Society
1
14
12
1
2
4
3
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
5
6
Fig. 2
Structures of twisted arenes
aromatic system (Fig.
2
). Similarly, 1,14-dimethyldibenzo[
c
,
g
]phenanthrene
(
3
) and related molecules can also be expected to possess a twisted dibenzo[
c
,
g
]
phenanthrene (pentahelicene) framework.
Highly substituted naphthalenes, such as octamethylnaphthalene (
4
), also exhibit
significant twists of the aromatic system [
11
,
12
]. The X-ray structure of
decaphenylanthracene (
5
) shows a large end-to-end twist [
13
]. Higher acenes
with overcrowded structures, such as octaphenyldibenzo[
a
,
c
]naphthacene
6
[
14
],
have also been reported to show high degrees of twist.
The twisted structure of 4,5-dimethylphenanthrene (
1
) contributes to the chiral-
ity of the molecule. It was recognized early on that the ability to resolve the two
enantiomers could provide supporting evidence for the nonplanarity of the aromatic
system [
1
]. Determinations of the rates of racemization and the activation barriers
of twisted chiral polyarenes have been actively pursued. Resolutions of the
enantiomers to allow these investigations to proceed were achieved in several
cases. In other cases, variable-temperature nuclear magnetic resonance (NMR)
experiments were employed to provide insights into the configurational stabilities
of the molecules. For practical applications, such as using these twisted compounds