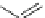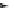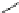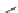Chemistry Reference
In-Depth Information
F
3
C
CF
3
F
F
O
O
F
F
F
F
F
O
O
F
69
R
3
Si
F
3
C
CF
3
SiR
3
F
3
C
CF
3
F
F
1. R
3
SiC
2
Li
2. SnCl
2
F
F
F
F
F
F
70
a
:R=
i-
Pr
b
: R=
t-
Bu
c
:R=cyclopentyl
R
3
Si
F
3
C
CF
3
SiR
3
R
3
Si
F
3
C
CF
3
SiR
3
O
2
70c
,O
2
F
F
F
F
O
O
F
F
F
F
71
, R = cyclopentyl
R
3
Si
F
3
C
CF
3
SiR
3
Fig. 32 Synthesis of crystalline nonacenes 70 and their endoperoxides 71 [
61
]
these compounds. While 70a adopted a 2D
ˀ
stacked motif, 70b and 70c show 1D
parallel “slipped” stacks [
61
]. The nonacene backbone is almost planar in 70b,but
it is warped in 70a and more so in 70c [
61
].
Dissolution of the crystals in toluene produces olive colored solutions of 70a-c
[
61
]. The measured UV/vis-NIR spectra (Fig.
34
) resemble those of parent
nonacene [
60
]. The authors stressed that nonacenes 70a-c do not show fluorescence
in the visible region (see below) [
61
]. The S
0
-S
1
transitions of silylethynyl
substituted acenes from anthracene to nonacene (without octacenes) are displayed
in Fig.
34
.
Note that Purushothaman et al. were unable to measure the
1
H NMR spectra of
their nonacene derivatives although they were sufficiently soluble [
61
]. The spectra
improved over time and signals due to the photodecomposition product appeared.
This decomposition product was independently generated and identified as the
endoperoxide on ring 4 (71) based on X-ray crystallography (Fig.
32
)[
61
]. Interest-
ingly, the pure endoperoxide 71 fragments in the LDI-TOF-MS to the
corresponding nonacene, and that is by far the most prominent mass signal [
61
].