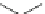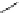Chemistry Reference
In-Depth Information
Fig. 30 Vis/NIR spectrum obtained after irradiation (
185 nm) of the nonacene sample
prepared as described in the caption of Fig.
29
. Reprinted with permission from [
60
]. Copyright
2010 John Wiley and Sons
λ ¼
O
O
67
O
O
O
>360n
m
68
Ar, 30 K
O
>305nm
9
Ar,30K
+/-
185 n
m
Ar, 30 K
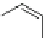
Fig. 31 Photochemical conversion of nonacene precursor 67 to nonacene 9 and its ionization to
the nonacene radical ions [
60
]
groups, of electron-withdrawing fluorine substituents at the terminal rings of the
aromatic core, and of two 3,5-di(trifluoromethyl)phenyl groups at the central ring
(Fig.
32
)[
61
].
The key to success was the crystallization of the nonacene derivatives 70 from
the reaction mixture of the final oxidation step. This approach avoided handling of
the very unstable compounds in solution for an extended period of time. The solids
thus obtained were stable when stored in the dark at 10
C over more than 2 days
[
61
]. The crystals were suitable for X-ray crystallography and this demonstrated the
presence of the nonacene aromatic core (Fig.
33
). The quality of the datasets does
not allow detailed bond lengths discussions, but reveals the stacking behavior of