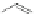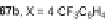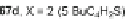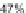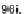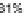Chemistry Reference
In-Depth Information
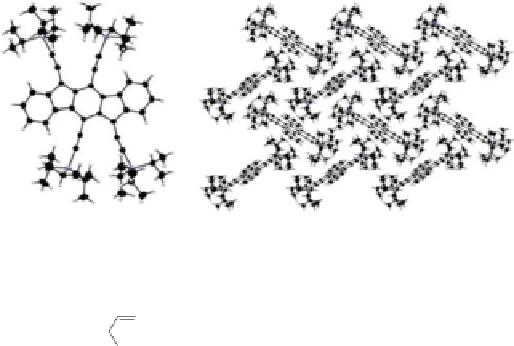
Fig. 8 X-Ray single crystal structure (
left
) and expanded herringbone crystal packing of 92a
(
right
)[
60
]
Fig. 9 Structures of Thiele's (93) and Tschitschibabin's (94) hydrocarbons [
74
]
Scheme 25 Synthesis of 6,12-diethynyl IFs 96a-i [
55
]
Examination of the crystal packing indicated that 92a exhibited an expanded
herringbone pattern often found in unsubstituted acenes [
75
]. This undesirable
packing motif is attributable to the steric bulk of the four interdigitated (triisopro-
pylsilyl)ethynyl groups in 92a.
Regarding the effect of ethynylation on the IF core, computational studies
suggested that ethynyl groups on the 5- and 11-positions had only a minor influence
on the overall electronics, as their removal changed the calculated HOMO and
LUMO energies by +0.02 eV and
0.10 eV, respectively [
55
]. Functionalization
of the favorable 6,12-diethynyl IF scaffold with withdrawing groups on the 2- and
8-positions further lowered the calculated HOMO and LUMO energies to levels
that approach those in the fullerene PCBM [
7
,
8
,
76
]. Ethynylation of 22, 61a-d,
and 67a-d using the lithiate of TIPSA generated mixtures of diol isomers, and
subsequent reduction using anhydrous SnCl
2
afforded fully conjugated indeno[1,2-
b
]fluorenes 96a-i in 31-81% yield over two steps (Scheme
25
).