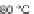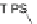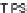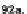Chemistry Reference
In-Depth Information
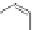
Scheme 24 Synthesis of fully conjugated 5,6,11,12-tetraethynyl IFs 92a,b [
60
]
tetraiodo framework 85a,b that Swager had already prepared [
60
]. Unfortunately,
direct Sonogashira cross-coupling of (trimethylsilyl)acetylene (TMSA) with 85a,b
did not afford the desired product. Instead, ethynylation of diones 65d and 90 with
the anion of (triisopropylsilyl)acetylene (TIPSA) gave diols 91a,b; subsequent
reduction using anhydrous SnCl
2
at elevated temperatures, a common technique
used in acene synthesis, afforded compounds 92a,b in 22% and 55% yield, respec-
tively, over two steps. As observed for 85a-c, 92a,b exhibit up-field aromatic
chemicals shifts with respect to 65d and 90, an indicator of their fully conjugated,
anti-aromatic state.
The absorption spectra of 92a,b showed three low-energy transitions in a pattern
with
l
max
values of 594 and 614 nm, respectively, which is roughly a 25 and 45 nm
bathochromic shift with respect to 85a due to the increased conjugation and lower
HOMO/LUMO gap caused by the four ethynyl groups. In comparison to 88, whose
low-energy transition
l
max
value is 644 nm, 92a,b were hypsochromically shifted
by 50 and 30 nm, a consequence of having two fewer
-electrons in the anti-
aromatic core. Unlike 88, however, 92a,b are non-emissive, a trait often observed
for antiaromatic molecules.
As with previously mentioned IF diones 65a-f, 67d,e, 73, and 76, cyclic
voltammetry data of 92a showed first and second reduction potentials of
p
0.62 V
and
1.16 V, respectively, while decyl IF 92b exhibited potentials of
0.73
and
1.29 V, respectively. Additionally, 92a,b do exhibit oxidation potentials
between 1.10 and 1.30 V, although this process is irreversible. Despite the lack
of electron-withdrawing diones, the first reduction potentials of 92a,b meet or
surpass the values of 65a-f, 67d,e, 73, and 76 because a twofold reduction of
the indeno[1,2-
b
]fluorene core is extremely favorable as the resultant dianion is a
22
-electron species where each ring is fully aromatic.
X-Ray analysis of 92a concluded that there was indeed long and short bond
alternation throughout the central portion of the IF core (Fig.
8
), which agrees with
Scherf's initial hypothesis regarding the internal bond structure of polymer 86 and
model compound 87a. Interestingly, the peripheral benzene rings remain
homogenized in length, suggesting that the name dibenzo-
s
-indacene is also a
valid descriptor. The bond lengths in the internal three rings show that 92a is
indeed a stable example of a molecule containing a
p
-xylylene core, similar to
what was found for Thiele's and Tschitschibabin's hydrocarbons (Fig.
9
)[
74
].
p