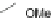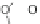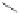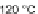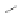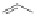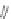Chemistry Reference
In-Depth Information
Scheme 14 Preparation of 2,8-dibromo IF dione 61c [
37
]
Fig. 3 Solid state packing of 57d [
51
]
Examination of the X-ray data for several [1,2-
b
]IF diones revealed that the
extent of halogenation had a dramatic effect on the solid state ordering. For
example, perfluoro 57d exhibited one-dimensional columnar stacking while
difluoro 61a demonstrated face-to-face
-stacking (Fig.
3
)[
51
,
52
]. Despite these
small structural differences, close contact distances for 57d and 61a were found to
be 3.31
˚
and 3.30
˚
, respectively.
Taking advantage of the fortuitous halogenation on the 5- and 11-positions
obtained via Swager's iodine-mediated transannular cyclization route (Scheme
13
),
Haley and coworkers appended a variety of trialkylsilylacetylenes onto 57a.
Sonogashira cross-coupling afforded the 5,11-diethynylindeno[1,2-
b
]fluorene-
6,12-diones 65a-f in 7-61% yield (Scheme
15
)[
53
]. Cyclic voltammetry data
illustrated the ability of 65a-f to accept two electrons reversibly. The half-wave
potential for the first reduction occurs at ca.
p
0.80 V, which is less negative than
that of parent 22 (
1.19 V) due to the electron-withdrawing acetylenes but more
negative compared to halogenated IF diones 61a-c (
0.7 V to
0.57 V) and 57d
(
0.45 V). Another advantage of silylacetylene incorporation was a significant
increase in solubility, given that minimal solubility in common organic solvents is a
hallmark characteristic of nearly all [1,2-
b
]IF diones.
While silyl substitution on the acetylene produced little variance in the optical
and electronic properties of 65a-f, it did have a significant effect on the solid state