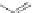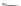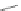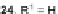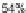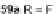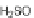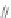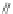Chemistry Reference
In-Depth Information
Scheme 12 Aerobic iodine-
induced transannular
cyclization of 57a-d [
49
,
50
]
Scheme 13 Preparation of
parent dione 22 and 2,8-
dihalo IF diones 61a,b
[
48
,
52
]
The most common route to IF diones is still based on Deuschels' original 1951
procedure [
25
]. In 2002, Wang and coworkers modified this synthesis, utilizing a
Suzuki cross-coupling reaction to generate terphenyl 20, which was then oxidized
and cyclized as before to yield 22 from 2,5-dibromo-
p
-xylene 58 in 85% yield over
three steps (Scheme
13
)[
48
]. Dione 22 could subsequently be reduced to form
known 6,12-dihydroindeno[1,2-
b
]fluorene (26).
Yamashita and colleagues synthesized 22 and 61a-c to investigate their utility
as n-type semiconducting materials in OFETs [
52
], inspired by the n-type behavior
Komatsu had observed with 57d [
51
]. Difluoro- and dichloroterphenyls 59a and
59b were formed via either Stille or Suzuki cross-coupling conditions from
58 (Scheme
13
). Oxidation with potassium permanganate generated dihalodiacids
60a,b and then cyclization onto the outside rings afforded dihalodiones 61a,b
in moderate yield. They assessed the performance of these substrates in HMDS-
treated OFETs (vapor deposition; bottom-contact; Si/SiO
2
). 61a exhibited the best
performance, with mobilities as high as 0.17 cm
2
/V
s and on/off ratio greater than
10
7
, while 22 exhibited no semiconducting behavior.
Marks showed that 2,8-dibromodione 61c could be made in a slightly
different manner (Scheme
14
)[
37
]. Suzuki cross-coupling of methyl 5-bromo-2-
iodobenzoate (62) with 1,4-dibenzenediboronic acid dipinacolate (63) afforded
diester 64; subsequent hydrolysis and cyclization onto the inside ring using
concentrated sulfuric acid gave 61c.