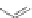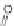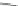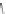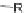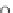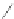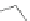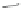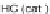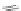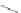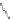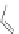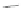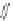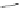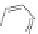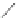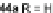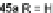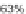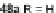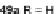Chemistry Reference
In-Depth Information
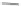
Scheme 10 Synthesis of
spiro-fused [1,2-
a
]IFs 48a,b
and [2,1-
c
]IFs 49a,b [
46
]
cyclohexenone 39. Reduction with sodium borohydride afforded cyclohexanol 40
followed by reaction with Pd/C to form 1,3,5-trimethyl-2,4-diphenylbenzene 41.
Oxidation with potassium permanganate, first using pyridine as the base yielding
diacid 42, and then using sodium carbonate, provided triacid 36. Cyclization in
concentrated sulfuric acid afforded 5-carboxyindeno[1,2-
a
]fluorene dione 43 in
near quantitative yield. Subsequent decarboxylation using elemental Cu at 350
C
gave 12 as the sole product.
2.2 Other Indeno[1,2-
a
]fluorenes
Since the initial work of Chardonnens and others, few examples of syntheses of
[1,2-
a
]IF derivatives exist outside of patent literature. Shi et al. recently reported
[1,2-
a
]IFs 48a,b and [2,1-
c
]IFs 49a,b (Scheme
10
)[
46
]. Diels-Alder reaction of
alkynes 45a,b, readily available from fluorenones 44a,b, provided regioisomeric
mixtures of diols 46a,b and 47a,b. Subsequent acid-catalyzed Friedel-Crafts
condensation gives 48 and 49. The two derivatives of both regioisomers showed
quantum yields in solution of ~60% and showed good thermal stability, with
decomposition occurring at temperatures greater than 330
C.
Minuti and colleagues described the syntheses of cyclophane-fused [1,2-
a
]IFs
[
47
]. Reduction of 50 with sodium borohydride, followed by treatment with PBr
3
then LiBr/LiCO
3
, gave three undetermined intermediates (Fig.
2
). This mixture of
molecules yielded 51 after treatment with Pd/C in triglyme at 160
C.