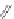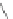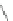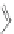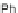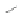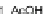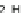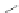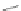Chemistry Reference
In-Depth Information
Fig. 2 Cyclophane-functionalized precursor 50 and [1,2-
a
]IF 51 [
47
]
Scheme 11 Synthesis of IF diones 55a,b [
48
]
3
Indeno[1,2-
b
]fluorenes
3.1
Indeno[1,2-
b
]fluorene-6,12-diones
Diels-Alder methods can also be used to generate the terphenyl core that ultimately
furnishes [1,2-
b
]IF diones. Wang showed that the [4+2] cycloaddition of cyclopen-
tadienone 52 with either diphenylacetylene (53a) or phenylacetylene (53b)
followed by cheletropic elimination of CO afforded diesters 54a,b (Scheme
11
)
[
48
]. Ester hydrolysis and subsequent Friedel-Crafts ring closure furnished 5,11-
diphenyl and 5-phenyl [1,2-
b
]IF diones 55a,b in 90% and 88% yield, respectively.
A noted consequence of this alternative pathway was the suppressed formation of
the corresponding [2,1-
c
]IF diones.
Encouraged by the potential semiconducting properties polyacetylenes could
offer, Swager reacted 24 and 56a,b [
49
], and later Komatsu reacted 56c [
50
,
51
],
with iodine under aerobic conditions to give 5,11-diiodoindeno[1,2-
b
]
fluorenediones 57a-d in 33-95% yield (Scheme
12
). The reactivity is reminiscent
of Eglinton's earlier transannular cyclization (Scheme
6
) but leading to diones in
this case due to the reactive nature of the fully-reduced dibenzo-
s
-indacene inter-
mediate (further discussed in Sect.
3.3
).
Komatsu and colleagues also demonstrated n-type semiconducting behavior
for 57d in thin-film OFETs [
51
], a highly desirable characteristic given the relative
paucity of organic n-type semiconducting scaffolds compared to p-type mole-
cular motifs. These devices, however, possessed low electron mobilities of
2.93
10
-5
cm
2
/V
10
-6
cm
2
/V
s under vacuum and 6.08
s in air.