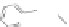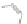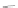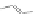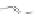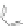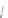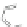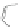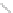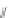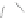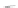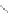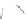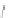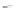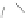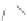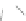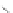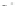Chemistry Reference
In-Depth Information
O
R
R
Cl
Cl
1)
Pd(PPh
3
)
2
Cl
2
/CuI,
NEt
3
, rt
2) K
2
CO
3
, THF/MeOH, rt
TMS
I
165
Cl
R
R
xylene, 160 °C,
μ
W
58%
85%
Cl
I
Ni(COD)
2
, COD,
2,2'-bipyridine,
toluene/DMF,
80 °C
Cl
Cl
163
164
81%
R
R
166
R
R
R
R
R
R
R
R
R
R
R
R
FeCl
3
,
CH
2
Cl
2
/
CH
3
NO
2
,
rt
92%
R
R
R
R
R
R
R
R
R
R
R
168
R= C
12
H
25
R
167
Scheme 43 Synthesis of nano-graphene using cross-coupling, Diels-Alder, and dehydrogenation
reactions [
107
]
3.1 Polymerization Reactions
The creation of polymer-type PAHs requires a suitable choice of building blocks.
M¨ llen and coworkers impressively demonstrated the synthesis of large nano-
graphenes by combining cross-coupling, Diels-Alder, and dehydrogenation
reactions (Scheme
43
)[
107
]. First, a building block containing two acetylene
units was prepared which was then subjected to Diels-Alder reaction with
cyclopentadienone. A Ni-promoted Ullmann coupling provided the polymer 167,
which is then planarized into a graphene ribbon 168 upon aromatization.
Suzuki cross-coupling reactions have also been applied for the synthesis of
graphene ribbons (Scheme
44
)[
108
]. In the example shown the
-bisboronic
ester 171 is polymerized to the intermediate 172. Final dehydrogenation yielded the
graphene ribbon 173.
para
3.2 Postfunctionalization of PAHs for the Extension
to Graphene
Most PAHs have only unreactive C-H bonds at the periphery. For further extension
the incorporation of synthetic handles is required to be able to make use of the tools
described above. Classical aromatic substitutions (e.g., halogenation reactions)
usually lack the selectivity required for efficient further synthetic elaborations.