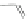
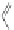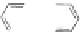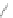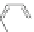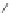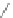Chemistry Reference
In-Depth Information
HO
OH
B
R
R
R
R
R
R
1)
n
-BuLi, -78°C
O
O
O
Br
I
I
Br
Br
B
B
O
Pd(PPh
3
)
4
,
aliquat 336, K
2
CO
3
toluene 80°C, 24h
93%
2)
O
O
B
O
R
R
R
R
R
R
171
170
169
82%
Pd(PPh
3
)
4
,
aliquat 336, K
2
CO
3
toluene 80°C, 24h
75%
R
R
R
R
R
R
R
R
FeCl
3
, CH
3
NO
2
Ph
Ph
65%
R
R
R
R
R
R
R
R
n
n
173
R= alkyl
172
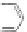
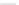
Scheme 44 Preparation of a nano-graphene ribbon via Suzuki cross-coupling reaction [
108
]
Bpin
Bpin
B
2
pin
2
(excess)
85 ºC, THF, 4 d
[Ir(OMe)COD]
2
(20%)
4,4'-dmbpy (40%)
t
BuOK (10%)
Bpin
Bpin
16
174
10-20%
Scheme 45 Selective Ir-catalyzed borylation of coronene 16 [
109
]
Recently, the group of Scott found that the direct borylation of PAHs proceeds in a
reversible manner, finally delivering the thermodynamically most stable isomer
[
109
]. With this methodology large PAHs can be selectively converted into
boronates, which are suitable reaction partners for further transformations, such
as Suzuki cross-coupling reactions (Scheme
45
).
3.3 Direct Extension of PAHs by Diels-Alder Reaction
In an ideal approach PAHs could be extended by simple addition of a C
2
H
2
-unit,
followed by oxidation to the conjugated aromatic species. Usually, aromatic
structures have been thought to be unreactive in such addition reactions. However,
in 2012 the group of Scott found during their studies towards the elongation of
carbon nanotube templates [
110
] that nitroethylene is a suitable dienophile in the