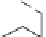Chemistry Reference
In-Depth Information
O
O
>395nm
PMMA
15
6
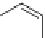
Fig. 5 Photochemical generation of hexacene from
α
-diketone 15 [
29
]
The electrical conductivity of hexacene under pressure was studied in 1964
[
26
]. It was found to resemble the properties of pentacene in as much as the
resistance decreased with pressure, but firm statements could not be made due to
the limited supply of hexacene [
26
]. The conductivity of doped hexacene thin films
was also investigated [
27
,
28
]. Minakata et al. [
27
] found that doping with alkali
metals, i.e., with electron donors, increased conductivity to 10
4
to 10
5
-1
cm
-1
,
but much less so than observed for pentacene films. Likewise, the conductivity
increased upon acceptor doping (iodine) [
28
]. The lower conductivity of doped
hexacene compared to pentacene films was ascribed to structural disorder of the
hexacene films, as these were amorphous while the pentacene films were crystal-
line. The hexacene thin films also showed optical properties that differed from
pentacene: the initially green thin film of hexacene lost color within seconds after
exposure to air, but upon doping with Rb the green color first regenerated and
eventually the yellow color of hexacene-Rb formed. The doped yellow material
turned gray during measurement of the UV/vis spectrum [
27
]. The iodine doped
hexacene film also has a yellow color and a new absorption peak at 360 nm [
28
].
Mondal et al. reported an investigation of the photochemical synthesis of
hexacene from an
Ω
-diketone 15 in 2007 (Fig.
5
)[
29
]. A similar procedure was
used successfully earlier for the photochemical formation of pentacene [
30
,
31
].
Photolysis of a degassed toluene solution of 15 produced the absorption spec-
trum of hexacene in the early stages of conversion. However, the hexacene
absorptions only increased initially; continued irradiation to effect a higher degree
of conversion of the
α
-diketone 15 produced a hexacene dimer and oxygen adducts
according to matrix assisted laser desorption ionization (MALDI) mass spectrome-
try [
29
]. The structure of the decomposition products could not be elucidated.
Photogeneration of hexacene in oxygen saturated solutions indicated the formation
of endoperoxides based on the typical singlet of the bridgehead protons between 6.0
and 6.1 ppm in the
1
H-NMR spectrum [
29
]. The formation of oxygenated products
indicates that hexacene in solution immediately reacts with trace amounts of
oxygen during irradiation.
Neckers and coworkers could, however, generate hexacene photochemically
after the photoprecursor had been embedded in a poly(methyl methacrylate) matrix
(PMMA) [
29
]. Due to immobilization of the molecules, this matrix prevents
photodimerization of hexacene and minimizes contact with ambient oxygen.
Within the PMMA film (
0.5 mm thick), hexacene is stable under ambient
conditions overnight, but it is slowly oxidized by diffusion of molecular oxygen
into the PMMA matrix [
29
].
α