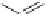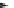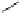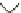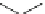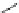Chemistry Reference
In-Depth Information
H
H
Cu
300-320 °C
H
H
10
6
Fig. 3 Synthesis of hexacene 6 from 5,16-dihydrohexacene 10 [
12
]
OH
O
OH
O
OHC
NaOH
+
OHC
OH
O
OH
O
11
12
13
H
H
Zn/H
2
CuO
270°C
0.15 Torr CO
2
370-390 °C
H
H
14
6
Fig. 4 Three-step synthesis of hexacene 6 [
17
]
made hexacene, but would not be able to publish this result for technical reasons.
Clar asked Marschalk to leave the investigation of hexacene to him. Marschalk
agreed a few weeks later (September 28, 1938), and Clar expressed his gratitude to
Marschalk in his paper.
A number of improved syntheses of hexacene have been reported since 1939
[
14
-
17
]. The improvements are related to the synthesis of dihydrohexacenes (like
14), and the final step remains the catalytic dehydrogenation. An example (Fig.
4
)
is the three-step synthesis reported by Satchell and Stacey [
17
] that starts with an
aldol condensation between 9,10-dihydroxyanthracene-1,4(2
H
,3
H
)-dione 11 and
naphthalene-2,3-dicarbaldehyde 12.
Due to its sensitivity to air and light, hexacene was not much investigated
experimentally in the following decades. An X-ray crystallography investigation of
small, extremely thin crystals that were invariably twinned allowed one to obtain the
space group P-1 and unit cell dimensions, but the poor quality of the data precluded
determination of bond parameters [
18
]. The absorption spectrum of hexacene
received most attention. It was studied for crystalline material by Preuss and
Zander [
19
], in solution by Wirz and co-workers [
20
] and Nijegorodov et al. [
21
],
and in a solid argon matrix by Bettinger et al. [
22
]. The Wirz group also obtained
solution spectra of the hexacene dication and dianion [
20
], as well as of its radical ions
in the early 1980s [
23
]. The latter were also studied under matrix isolation conditions
in solid argon [
22
]. The infrared spectrum of hexacene is also available [
22
]. Photo-
electron spectroscopy studies have revealed the ionization potentials of hexacene
[
24
,
25
]. Biermann and Schmidt measured the second-order rate constant for the
Diels-Alder reaction with maleic anhydride in 1980 (see above) [
6
].