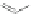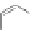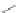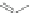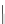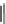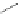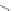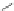Chemistry Reference
In-Depth Information
TMS
I
TMS
TMS
TMS
I
I
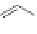
1) ICl, 92%
2)FeCl
3
, qua
n
t.
Co
2
(CO)
8
125°C
92%
TMS
TMS
I
I
TMS
TMS
I
91
92
93
Scheme 21 Synthesis of a substituted HBC 93 allowing further functionalization via Sonogashira
cross-coupling reactions [
51
,
52
]
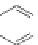
O
O
R
R
R
R
FeCl
3
O
O
O
O
O
Co
2
(CO
)
8
R
R
95a
R=alkyl
+
96
O
R
R
O
94
O
R
R
95b
Scheme 22 Synthesis of C
3
symmetric HBCs 96 [
55
]
-trimethoxy substituted hexaphenylbenzene pre-
cursor 95 with three alternating methoxy and alkyl (dodecyl) substituents was
synthesized and planarized by cyclodehydrogenation (Scheme
22
)[
55
]. A strong
aggregation in solution was also observed (by NMR spectroscopy). In the solid state
a complex helical superstructure was formed due to the influence of the methoxy
groups in
Similarly, a C
3
symmetric
meta
position as well as intermolecular interactions. After deposition on a
surface, microfibers were obtained.
meta