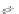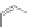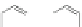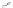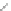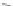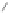Chemistry Reference
In-Depth Information
O
O
O
O
O
O
O
N
N
N
N
N
N
N
O
Co
2
(CO
)
8
O
O
N
N
N
N
N
N
N
O
O
O
O
85
86
87
20%
60%
Scheme 19 Cyclotrimerization of double substituted tolane 85 [
49
]
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
Co
2
(CO)
8
FeC
l
3
O
O
O
O
O
O
O
O
100°C
O
O
52%
O
O
88
O
O
O
O
O
O
O
O
O
O
O
O
O
O
89
90
Scheme 20 Synthesis of methoxy substituted HBC 90 [
50
]
hindrance between the methoxy groups at the periphery. X-Ray analysis of the
complex of this 90 with fullerene or hexafluorobenzene illustrated the ability of this
molecule to undergo host-guest chemistry.
A useful HBC building block has been designed for further functionalization,
hexa(4-iodophenyl)-
-hexabenzocoronene 93. This compound was also
prepared via cyclotrimerization of the silyl-protected alkyne 91. With this iodo-
HBC 93 further functionalization is possible despite its insolubility. Various func-
tional groups can be attached via Sonogashira cross-coupling reactions, giving a
starting point to a large series of compounds which form highly ordered columnar
liquid crystals (Scheme
21
)[
51
,
52
].
Introduction of long ethylene glycol chains lead to an amphiphilic HBC
[
53
]. This water soluble molecule can be used as a template for the fabrication of
porous silica with defined nano-channels.
C
3
symmetric HBCs with alternating polar and apolar substituents can also be
synthesized by statistical cyclotrimerization reaction of differently substituted
diphenylacetylenes (e.g., ester vs. alkyl). The reaction produced a mixture of two
isomers that were separated by column chromatography [
54
]. The synthesis was
completed by cyclodehydrogenation to planarize the system. This substitution
pattern has an influence on the packing mode, as the symmetry plays an important
role in the self-assembling process.
peri