Environmental Engineering Reference
In-Depth Information
Pairs of enantiomers are often described as right- and left-handed. To name the
enantiomers of a compound unambiguously, their names must include the handed-
ness of the molecule according to the R,S nomenclature (Cahn-Ingold-Prelog
rules). Enantiomers have, when present in a symmetric environment, identical
chemical and physical properties except for their interaction with polarized light
(so-called optical activity). For example, an enantiomer will absorb left- and
right-circularly polarized light to differing degrees, which is the basis of circular
dichroism spectroscopy. To illustrate the three-dimensional structure of a chiral
molecule by a two-dimensional drawing, one can use the projection already intro-
duced by Emil Fischer in 1891. It is still in use today, particularly in the case of
carbohydrates and amino acids [
21
].
Proteins are all exclusively made of a series of L-amino acids, whose order
dictates the primary structure. This homochirality leads to homochirality in higher-
order structures such as the right-handed
-helix found in some secondary struc-
tures or the way in which some proteins are folded to originate its tertiary structure
(Figure
3
). Table
2
presents the side-chain structures of the twenty standard
amino acids.
α
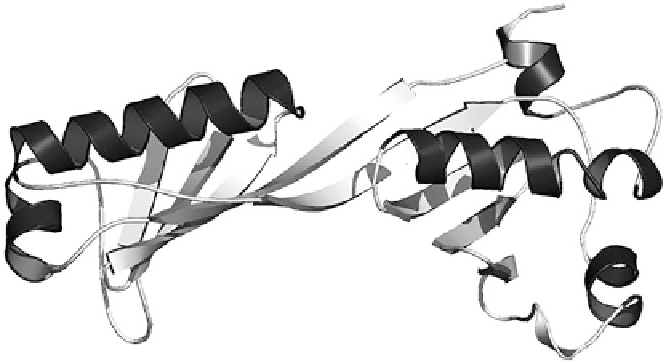
Figure 3 Ribbon diagram for the tertiary structure of the TATA binding protein from
Taenia
solium
showing its right-handed
α
helices (in black). Redrawn from [
48
] by permission of
R. Miranda.
On the other hand, nucleic acids consist of chains of deoxyribonucleosides (for
deoxyribonucleic acid, DNA) or ribonucleosides (for ribonucleic acid, RNA),
connected by phosphodiester bonds, all based exclusively on the D-deoxyribose
or D-ribose sugar ring, respectively [
22
]. The homochirality in the monomeric
sugar building blocks of nucleic acids leads to homochirality in their secondary
structures such as the right-handed B-type DNA double helix, as shown in Figure
4
.
Search WWH ::

Custom Search