Environmental Engineering Reference
In-Depth Information
Clays have a particular place in studies related to enzyme immobilization, protein
fractionation, soil ecosystem safety, genetic engineering, and specifically in the
biochemical evolution and origin of life on Earth. In 1949, Bernal suggested that
clay minerals played a key role in chemical evolution and the origins of life because
of their uptake capability, their ability to protect against ultraviolet radiation, to
concentrate, and to catalyze the polymerization of organic molecules [
14
]. A number
of subsequent reports supported this idea. It was claimed that clay minerals such as
montmorillonite, might have played a central role in the formation of proteins and
nucleic acids serving as primitive templates to concentrate the primordial biomole-
cules and to catalyze their polymerization. Furthermore, they helped in the preser-
vation of the first biopolymers that eventually initiated the biological evolution on
Earth [
15
-
20
].
3 Clays as Possible Catalysts in the Synthesis
of Biomolecules
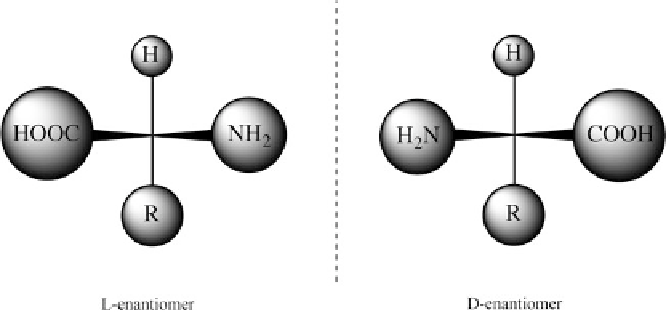
A distinctive feature of life's chemistry is its homochirality illustrated by amino
acids and sugars, fundamental biomolecules for the construction of proteins
and nucleic acids. A chiral molecule is a type of molecule that has a
non-superimposable relationship with its mirror image. The feature that is most
often the cause of chirality in molecules is the presence of one or several asym-
metric carbon atoms. Chiral molecules can exist in two distinguishable mirror-
image forms, designated as L- or D-enantiomers (optical isomers) (Figure
2
).
Figure 2 The two enantiomers of a generic
side-chain,
see Table
2
); L,D assignment is done with reference to glyceraldehyde, HO-CH(CH
2
OH)-CHO.
The structures are drawn as chemical standard structures, not as the typical zwitterionic forms that
usually exist in aqueous solution.
-amino acid, H
2
N-CH(R)-COOH (R
α
¼
Search WWH ::

Custom Search