Environmental Engineering Reference
In-Depth Information
CN
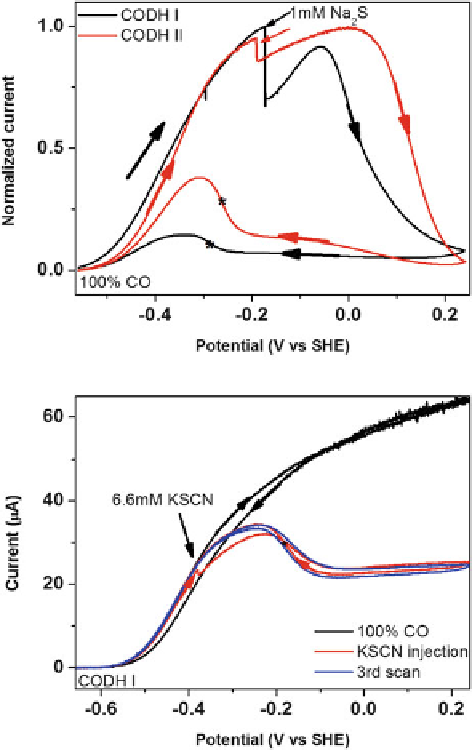
, but instead reacts to stabilize an inactive state at a higher oxidation level
(Figure
9
). This state must be analogous to but not identical with C
ox
because the
reactivation potential of the
-sulfido product is much more negative. It is therefore
unlikely that sulfide can be an activator of CODH catalysis, as was suggested by
earlier work [
33
,
40
]. An interesting observation is made when thiocyanate, NCS
,
is introduced. In marked contrast to cyanate, NCO
, NCS
does not inhibit CO
2
reduction; instead there is partial inhibition of CO oxidation and an oxidized
inactive state is formed that does not activate at the same potential as C
ox
(in CODH I), but at a more negative potential, closer to that observed when sulfide
is present. One possibility is that NCS
also reacts to stabilize an inactive oxidized
state by leaving a bridging sulfur, either as sulfide or an intact S-bound NCS
.
μ
Figure 9 Reactions of
CODH with sulfide and
thiocyanate. Upper Panel:
Cyclic voltammograms
showing the reaction of
CODH I
Ch
and CODH II
Ch
with sulfide: An aliquot of
Na
2
S stock solution (giving
1 mM final concentration)
was injected into the
electrochemical cell. The
panel is reconstructed using
data from [
18
] and
[
19
]. Lower Panel: Cyclic
voltammograms showing
the inhibition of CODH I
Ch
by thiocyanate: An aliquot
of KSCN stock solution
(giving 6.6 mM final
concentration) was injected
into the electrochemical
cell. Experimental
conditions: 25
C, 0.2 M
MES buffer (pH 7.0),
rotation rate 3500 rpm, scan
rate: 1 mV s
1
.
Search WWH ::

Custom Search