Environmental Engineering Reference
In-Depth Information
The main differences between CODH I
Ch
and CODH II
Ch
are the stronger
inhibition of CODH II
Ch
by CO product and cyanide [
19
]. These discoveries may
shed light on the possible role of CODH II
Ch
in biological systems. Interestingly,
sulfide is a similar inhibitor of [NiFe]-hydrogenases, binding to and stabilizing the
inactive Ni(III) form rather than acting competitively in the active potential region.
One aspect here is that sulfide is a good bridging ligand, which CN
is not, so it can
form the Ni-S-Fe linkage that is observed in some crystal structures.
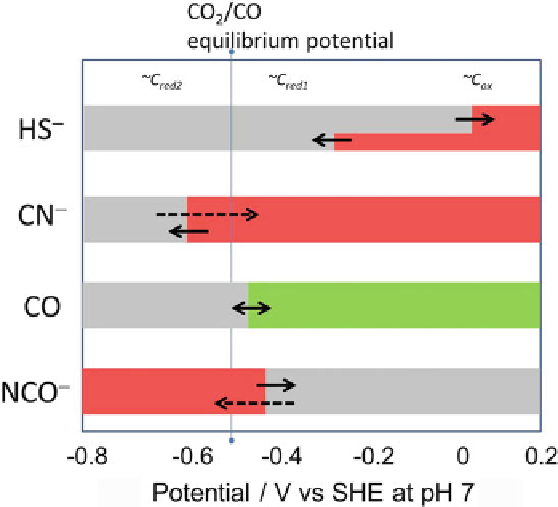
Figure
10
summarizes the 'redox spectrum' for activities and inhibition of
CODH I
Ch
.
Figure 10 Potential dependence of binding of inhibitors to CODH I
Ch
. In red potential regions,
the agent is an inhibitor. In green potential zones, the agent is a substrate that is transformed. In
gray zones, there is little or no interaction. The filled arrows indicate faster reactions compared to
those indicated by dashed arrows. Reprinted with permission from [
18
]; copyright 2013 American
Chemical Society.
6 Demonstrations of Technological Significance
The high electrocatalytic activities of Class IV CODHs match those observed with
many hydrogenases - a fact that inspired an aesthetically interesting experiment to
mimic the physiological process in which CO oxidation is coupled to H
2
evolution
[
59
]. This process is the biological counterpart of the industrially important 'water
gas shift' reaction that requires high temperatures. Notably, CO as a component of
syngas, can be used to synthesize liquid hydrocarbons by reaction with H
2
in the
Fischer-Tropsch process [
60
]. In the enzyme-coupled version, a hydrogenase (Hyd-2)
Search WWH ::

Custom Search