Biomedical Engineering Reference
In-Depth Information
D
D
×
10
−
6
×
10
−
6
D
D
7
7
6
6
5
Ubl TMP
5
Galactoglucomannan
4
4
3
3
Bl TMP
2
2
Xylan
1
1
Pectin
0
0
0
−
20
−
40
−
60
−
80
−
100
−
120
−
140
−
160
0
−
20
−
40
−
60
−
80
−
100
−
120
−
140
−
160
D
f
(Hz)
D
f
(Hz)
(a)
(b)
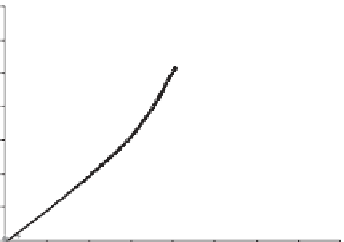
Figure 6.1
Change in dissipation vs. change in frequency for adsorption of 100 mg l
−1
hemicellulosesolutionsoncellulose. (a)Dissolvedhemicellulosesisolatedfromunbleached
TMP(UblTMP)andperoxidebleachedTMP(BlTMP)(b)purehemicelluloses.Hemicelluloses
were in10mMNaAc/HAcbuffer at pH5.6, except xylanwhichwas in1mMNaCl at pH
10. f
0
=
5MHz,n
=
3, t
=
200min,exceptt
=
100minforpectinandBlTMP.
10
−
6
D
D
×
7
Ubl TMP
6
5
Xylan
4
3
2
1
0
0
−
20
−
40
−
60
−
80
−
100
−
120
−
140
−
160
D
f
(Hz)
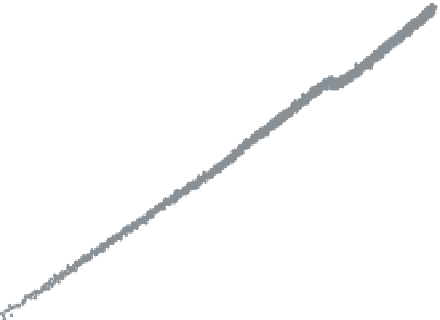
Figure 6.2
Change in dissipation vs. change in frequency for adsorption of 100 mg l
−1
solutions of dissolved hemicelluloses (10 mM NaAc/HAc buffer, pH 5.6, 100 mM NaCl)
and xylan (pH10, 10mMNaCl) at high ionic strengthon cellulose. Ubl TMP
=
dissolved
hemicellulosesisolatedfromunbleachedTMP. f
0
=
5MHz,n
=
3, t
=
200.











Search WWH ::

Custom Search