Chemistry Reference
In-Depth Information
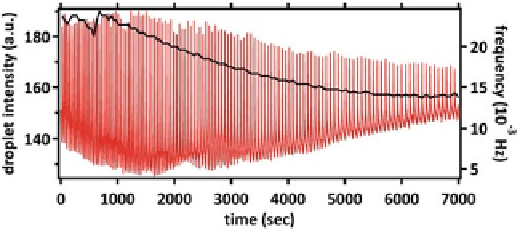
Fig. 5.4
BZ oscillations in a closed reactor. The oscillation trace and its corresponding frequency
(
black
) are plotted as a function of time
be considered constant. As mentioned before, the BZ oscillations display two time
scales, characteristic of a relaxation oscillator. The fast time scale in the order of 1 s
corresponds to the rapid autocatalytic production of HBrO
2
. The frequency of the
oscillation is then set by the slow production of bromine resulting the the decay of the
droplet intensity. Due to the presence of the surfactant mono-olein in our system, the
bromine cycle is modified from the classical version of the BZ reaction and therefore,
we see an effective decrease of frequency for the oscillator droplets compared to the
experiments in bulk.
The BZ oscillators are suspended in an oil phase consisting of squalane, with
mono-olein at concentrations well above the critical micelle concentration (CMC).
The mono-olein serves two purposes. First, it forms dense surfactant layers at the
the C
C double bond in the mono-olein molecule acts as an efficient scavenger for
bromine, since the latter rapidly reacts with this site. The oil phase is thus expected
to efficiently suppress coupling between neighboring droplets, which is mediated by
the exitatory and the inhibitory species as described earlier. That this is indeed the
case can be seen in Fig.
5.5
which shows a two dimensional hexagonal packing of BZ
oscillators. The spherical shape of the droplets in the packing clearly shows that there
is oil between the droplets and that bilayer membranes have not formed which are
characterised by increased contact angle of the surfactant monolayers as described
Fig.
5.5
and in our experiments we do not see a systematic dependance of the droplet
size on the oscillation frequency. This could be because for a certain concentration
of the BZ educts, as long as the droplet size is below the typical wavelength of
the BZ wave, there should not be a change in the temporal frequency. When such
isolated droplets are close to each other, in spite of the fact that the diffusion of the
excitatory and inhibitory species can indeed cause coupling between droplet, they
remain uncoupled. This is due to the fact that as we discussed before, they might be
trapped via a reaction with the surfactant molecules.
However, bilayer membranes form spontaneously between the droplets as
described in Chap.
2
and this happens in the case of the droplet oscillators too. As
=