Chemistry Reference
In-Depth Information
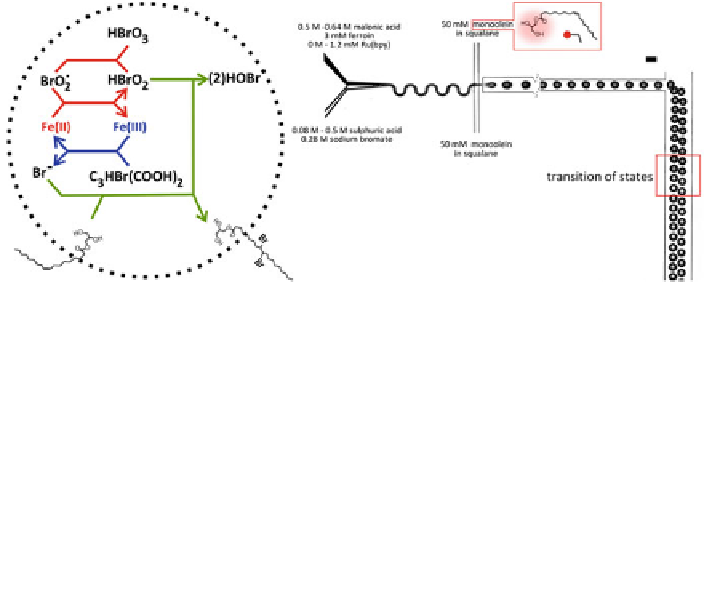
Fig. 5.1
Production of monodisperse oscillator droplets.
Left
The BZ reaction consists of two loops
(i) an autocatalytic and (ii) an inhibitory cycle. The reaction state can be visualised by the colour of
the ferroin catalyst. In our setting, an additional reaction with the unsaturated mono-olein surfactant
occurs as shown.
Right
The contents of the BZ reaction are mixed on the microfluidic chip to prevent
any pre-reaction. Seen through an optical 480/20 nm notch filter, the transition from
red colour
to
the
blue colour
of the BZ reaction is seen by the change in the brightness of the droplet
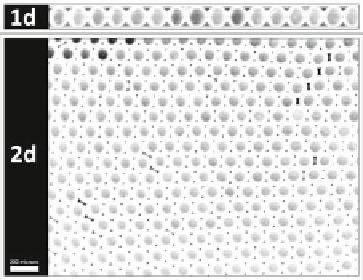
Fig. 5.2
Storage of droplet oscillations in one and two dimensional confinement for observation
of their dynamics
of the catalyst back from the blue colour to red. In addition to this classical BZ
reaction, in our case, due to the addition of the surfactant mono-olein to stabilise the
droplets an additional side reaction occurs. Since the surfactant has an unsaturated
hydrocarbon chain as shown, some of the bromine that is produced in the inhibitory
cycle rapidly reacts with the unsaturated bond. As we will see below, this 'trapping'
of the bromine by the surfactant significantly affects the coupling between droplet
oscillators in our setup.
The droplet oscillators are stored as a monolayer in either a 1d or 2d array as
shown in Fig.
5.2
. The 1d array is created within a glass capillary with a square
cross-section of inner width 100
m (Hilgenberg GmbH,
Germany). The inner walls of the capillary are hydrophobised using a commerically
µ
m and outer width 135
µ