Chemistry Reference
In-Depth Information
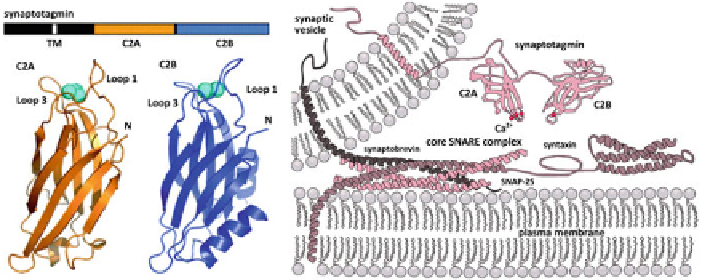
Fig. 3.2
Synaptotagmin-1 is the
Ca
2
+
sensor for membrane fusion.
Left
Synaptotagmin has a
single transmembrane domain (TM) which embeds into the lipid bilayer of the synaptic vesicle.
This is followed by a large cytoplasmic domain, which 'sticks out' into the cytoplasm of the cell.
This domain is comprised of two parts the C2A and C2B which have 2 and 3 sites, respectively, for
binding
Ca
2
+
ions.
Right
By the current understanding of the fusion process, the SNARE proteins
are nucleated i.e. they are primed for fusion via coiling but fusion is somehow arrested. In this
picture of fusion, the synaptotagmin acts downstream of the SNARE coiling (Reproduced with
permission from [
15
])
are already close enough to zipper even before the
Ca
2
+
influx, then membrane
fusion should proceed rapidly by the SNARE action. If they are not close enough,
then what are the mechanisms by which they stay apart? And how exactly does the
interplay of
Ca
2
+
and synaptotagmin-1 bring them close enough for fusion to occur
rapidly? In this chapter we seek to answer some of these questions. We show that
the electrostatic repulsion between the lipid membranes due to their charged lipid
components, is sufficient to keep membranes too far apart for fusion to occur. We
then show that the conformational change in synaptotagmin-1 due to the binding of
Ca
2
+
helps to overcome this electrostatic repulsion, thus bringing the membranes
close enough for fusion. First, we present a description of the current understanding
of synaptotagmin-1 and its role in membrane fusion.
3.1.1 Current Understanding of the Role of Synaptotagmin-1
Membrane binding of
Ca
2
+
-synaptotagmin-1 has recently been proposed to locally
increase membrane curvature, suggesting that synaptotagmin-1 might act by increas-
ing the membrane tension at the sites of fusion [
16
,
17
]. Synaptotagmin-1 also binds
directly to SNAP-25, syntaxin-1A, and the binary and ternary SNARE complexes
[
4
,
18
]. Some of the earlier studies showed that the polybasic patch and the
Ca
2
+
-binding sites bind to the SNAREs, but the relevance of this observation was
questioned since these are the same regions that bind to lipid membranes [
8
,
19
,
20
].
This discrepancy was recently overcome by a single molecule Förster Resonance
Energy Transfer (FRET) study showing binding of the C2B domain to the ternary